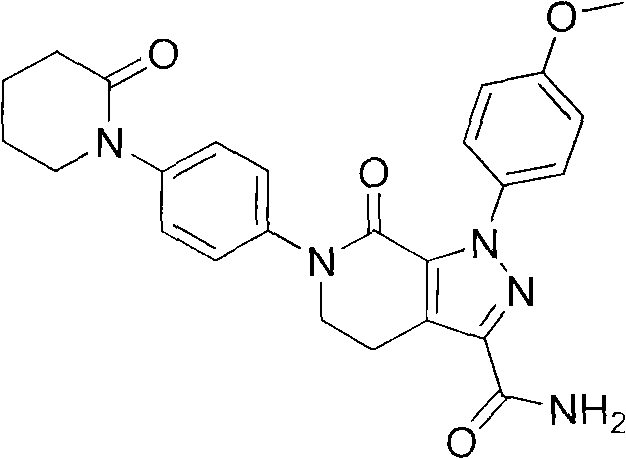
Synthetic method of apixaban intermediate 1-(4-nitrobenzophenone)-2-piperidone
A technology of nitrophenyl and apixaban, which is applied in the field of synthesis of apixaban intermediate 1--2-piperidone, can solve problems such as existing risks, and achieve low production cost and great economic benefits , the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
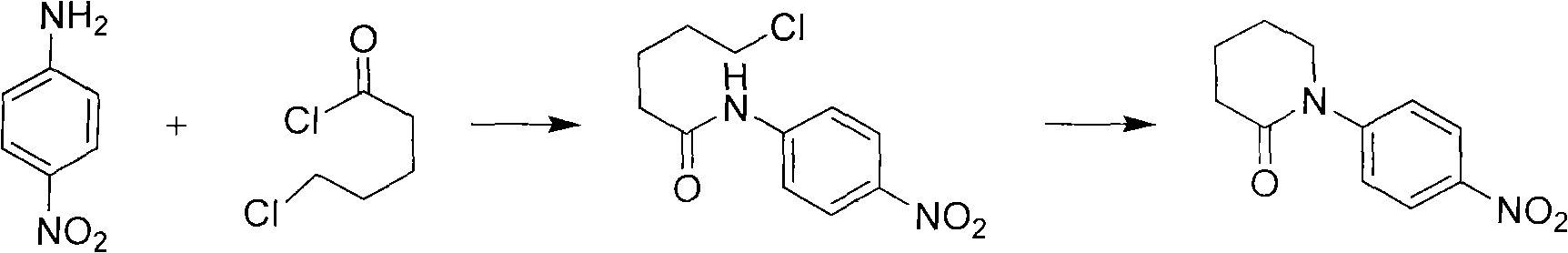
[0023] Synthesis of 1-(4-nitrophenyl)-2-piperidone
[0024] Dissolve 15 (0.1mol) of p-nitroaniline in 300ml of dichloromethane, add the prepared alkali solution (12g of sodium hydroxide + 150ml of water), add 0.75g of tetrabutylammonium bromide, stir vigorously, drop 60g of 5-chloropentanoyl chloride was kept at 25°C for 16 hours. The TLC reaction was completed, and the layers were separated. The organic phase was washed with 100 ml of water, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain a yellow solid, which was then recrystallized with ethyl acetate to obtain 19.8 g of yellow crystals, with a yield of 90%.
[0025] 1 HNMR (400MHz, CDCl 3 , ppm) δ: 8.27(d, J=8.7Hz, 2H), 7.52(d, J=8.7Hz, 2H), 3.75(t, J=5.9Hz, 2H), 2.64(t, J=6.4Hz, 2H), 1.95-2.02(m, 4H).
Embodiment 2
[0027] Synthesis of 1-(4-nitrophenyl)-2-piperidone
[0028] Dissolve 150g (1mol) of p-nitroaniline in 3000ml of chloroform, add the prepared alkali solution (168g of sodium hydroxide + 1500ml of water), add 6g of tetrabutylammonium bromide, and add dropwise 600g of 5-chloro Valeryl chloride, kept at 25°C for 16 hours. The TLC reaction was completed, and the layers were separated. The organic phase was washed with 1000 ml of water, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain a yellow solid, which was then beaten with methyl tert-butyl ether to obtain 193.8 g of yellow crystals, with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com