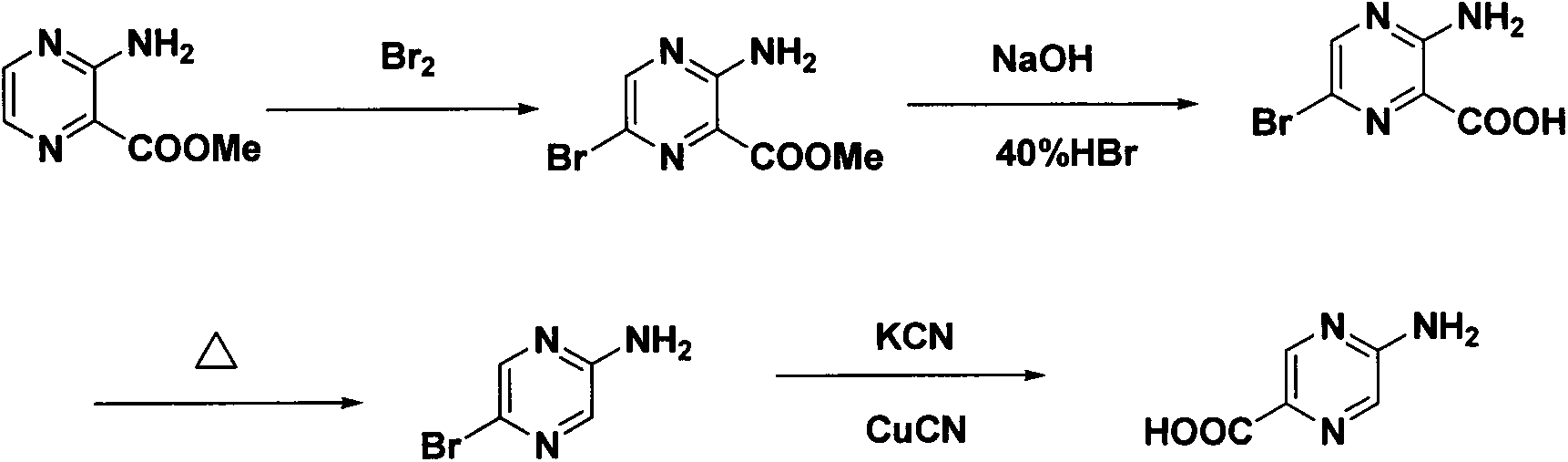
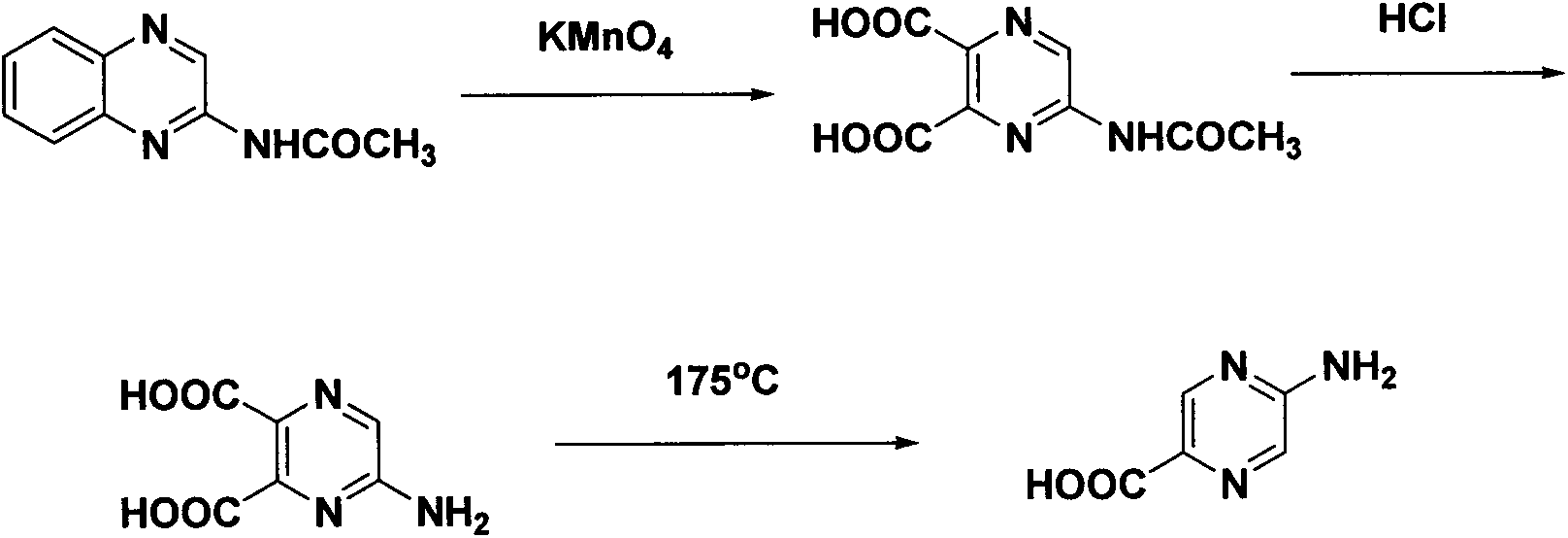
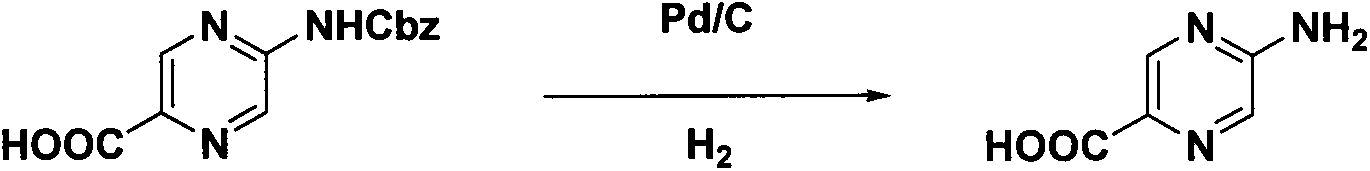Synthesis method of 2-aminopyrazinyl-5-formic acid
The technology of an aminopyrazine and a synthesis method is applied in the new synthesis process field of 2-aminopyrazine-5-carboxylic acid, and can solve the problems of difficult preparation of starting materials, unsuitable for scale-up production, high temperature required for the reaction, etc. The effect of shortening the production process, less impurities and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Synthesis of ethyl 2-formate-5-formylhydrazine pyrazine (compound 2)
[0027] 2,5-ethyl pyrazine dicarboxylate (85g, 0.38 mol) was dissolved in absolute ethanol (1500 ml), and under ice-bath conditions, 85% hydrazine hydrate (22.3g, 0.38 mol) was slowly added dropwise, and the addition was completed Stir overnight at room temperature, filter with suction, wash with absolute ethanol (100 ml), and dry in vacuo to obtain 60 g of white solid compound (2), yield: 75.3%. mp: 142-143°C
[0028] HNMR (DMSO-d6, 400MHz): δ9.47(1H, s), 9.23(1H, s), 8.84(1H, s), 4.55(2H, m), 4.16(2H, m), 1.47(3H, t).
Embodiment 2
[0029] Embodiment 2: Synthesis of ethyl 2-formate-5-formyl azidopyrazine (compound 3)
[0030] 2-Ethyl formate-5-formylhydrazine pyrazine (37g, 0.18 moles), was added dichloromethane (600 milliliters), water (600 milliliters), sodium nitrite (60g, 0.87 moles), under ice water cooling, 6N HCl (250 ml) was added dropwise. After the addition, the aqueous phase was extracted with dichloromethane (200 ml x 3), the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness with a water pump at room temperature to obtain off-white Solid compound (3) 33g, yield: 82.9%.
Embodiment 3
[0031] Embodiment 3: Synthesis of ethyl 2-formate-5-Boc-amino-pyrazine (compound 4)
[0032] 2-Ethyl formate-5-formylazide pyrazine (100g, 0.45 mol), add toluene (600ml) and t-BuOH (100ml), heat to reflux, react for 1 hour, cool to room temperature, and filter with suction , washed with toluene (50 ml), and dried to obtain 110 g of white solid compound (4), yield: 91.6%. mp: 162-163°C
[0033] HNMR (DMSO-d6, 400MHz): δ9.40(1H, s), 8.95(1H, s), 4.49(2H, m), 1.57(9H, s), 1.44(3H, t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com