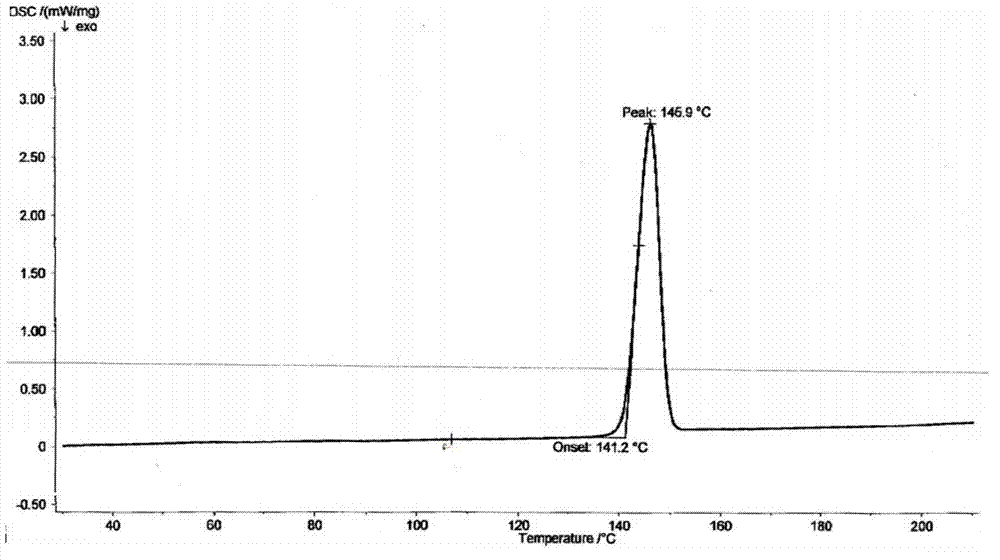
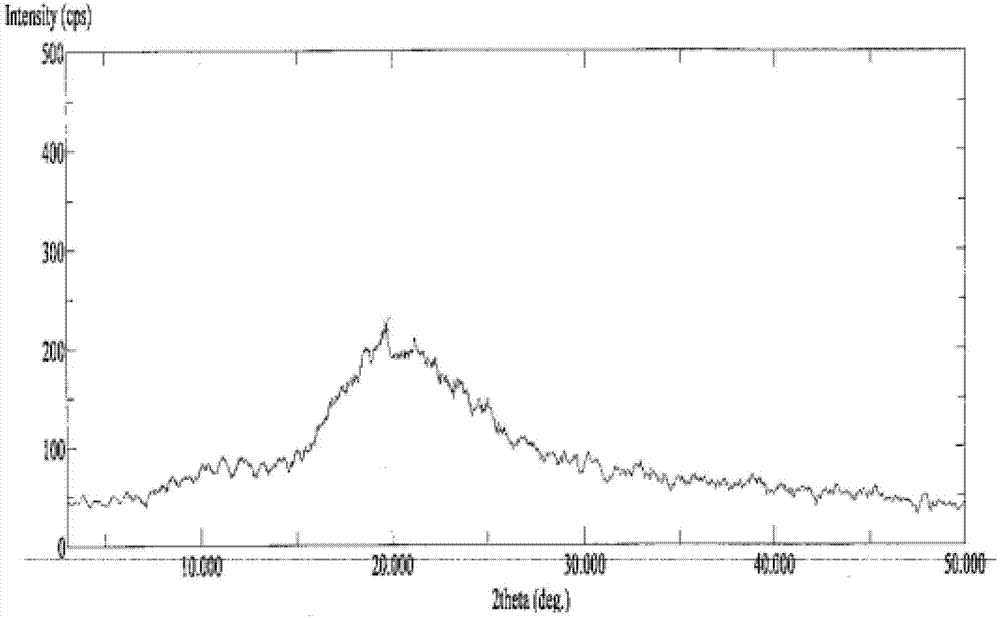
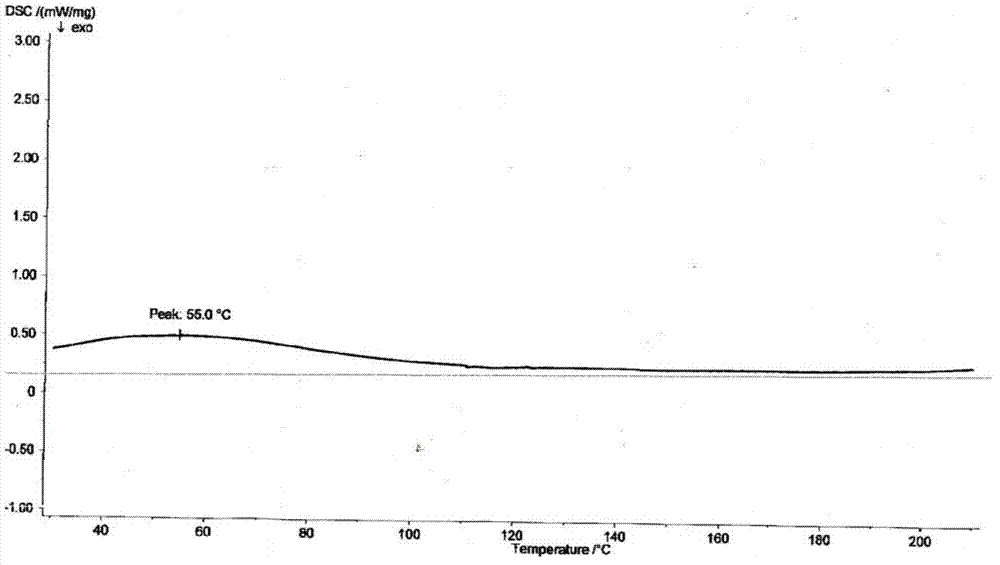
Dronedarone or powder of salt thereof and pharmaceutical composition prepared therefrom
A technology of dronedarone and dronedarone hydrochloride, which is applied in the field of antiarrhythmic drugs, can solve problems such as the dissolution rate of dronedarone hydrochloride, the influence of drug release and stability, and the difficulty in controlling the properties of mixture excipients. Facilitate the preparation operation, improve the dissolution rate and solubility, and the effect of good dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add soybean lecithin, Tween-20, Tween-80, lauric acid macrogol glyceride in the weight ratio as shown in Table 1 based on the weight of dronedarone hydrochloride, dissolve in the phosphate buffer solution of pH 4.5 to prepare. A solution containing 2 mg / mL of dronedarone hydrochloride was prepared, incubated at 37 °C for 2 hours, then the solution was diluted 10 times with neutral phosphate buffer, and the final pH of the solution was 6.8. The solution was filtered with a filter, and the solution was measured with a UV spectrophotometer at a wavelength of 217 nm. The absorbance value A of the solution was measured at a wavelength of 217 nm; another pH 4.5 phosphate buffer was taken to make a solution containing 2 mg / mL of dronedarone hydrochloride, and the solution was incubated at 37 °C for 2 hours. Then the solution was diluted 10 times with phosphate buffer of pH 4.5, the final pH of the solution was 4.5, after incubation at 37 °C for 2 hours, filtered through a 5 μm ...
Embodiment 2
[0045] Example 2 Dronedarone hydrochloride powder
[0046] 42.6 g of dronedarone hydrochloride and 4 g of soybean lecithin were dispersed in 500 mL of 70% ethanol, and spray-dried with a spray dryer to obtain powder. (wherein, the weight of soybean lecithin accounts for about 8.6% of the powder weight)
[0047] Spray drying conditions: spray dryer; nitrogen flow pressure: 600L / h; air inlet temperature: 90°C; air outlet temperature: 43°C; feed volume: 5mL / min; needle penetration frequency: 15 seconds / time.
Embodiment 3
[0048] Example 3 Dronedarone hydrochloride powder
[0049] 42.6 g of dronedarone hydrochloride and Tween-804 g were dispersed in 500 mL of absolute ethanol, and spray-dried with a spray dryer to obtain powder. (The weight of Tween-80 accounts for about 8.6% of the powder weight)
[0050] Spray drying conditions: spray dryer; nitrogen flow pressure: 1200L / h; air inlet temperature: 80°C; air outlet temperature: 32°C; feed volume: 15mL / min; needle frequency: 30 seconds / time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com