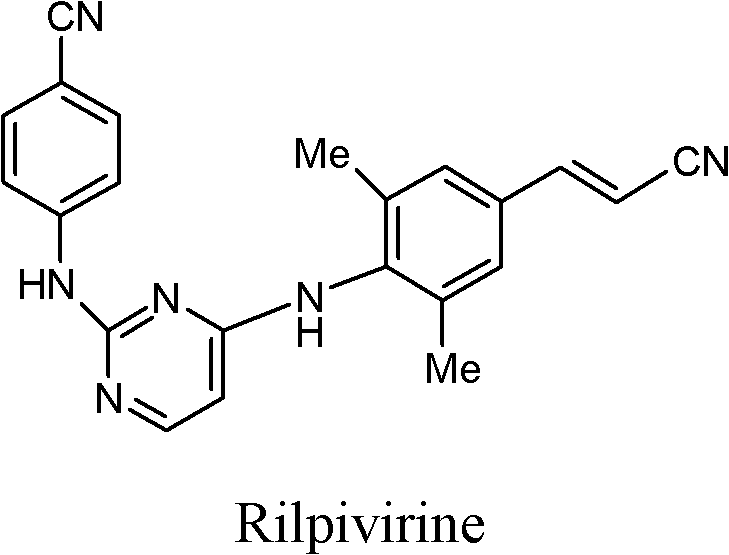
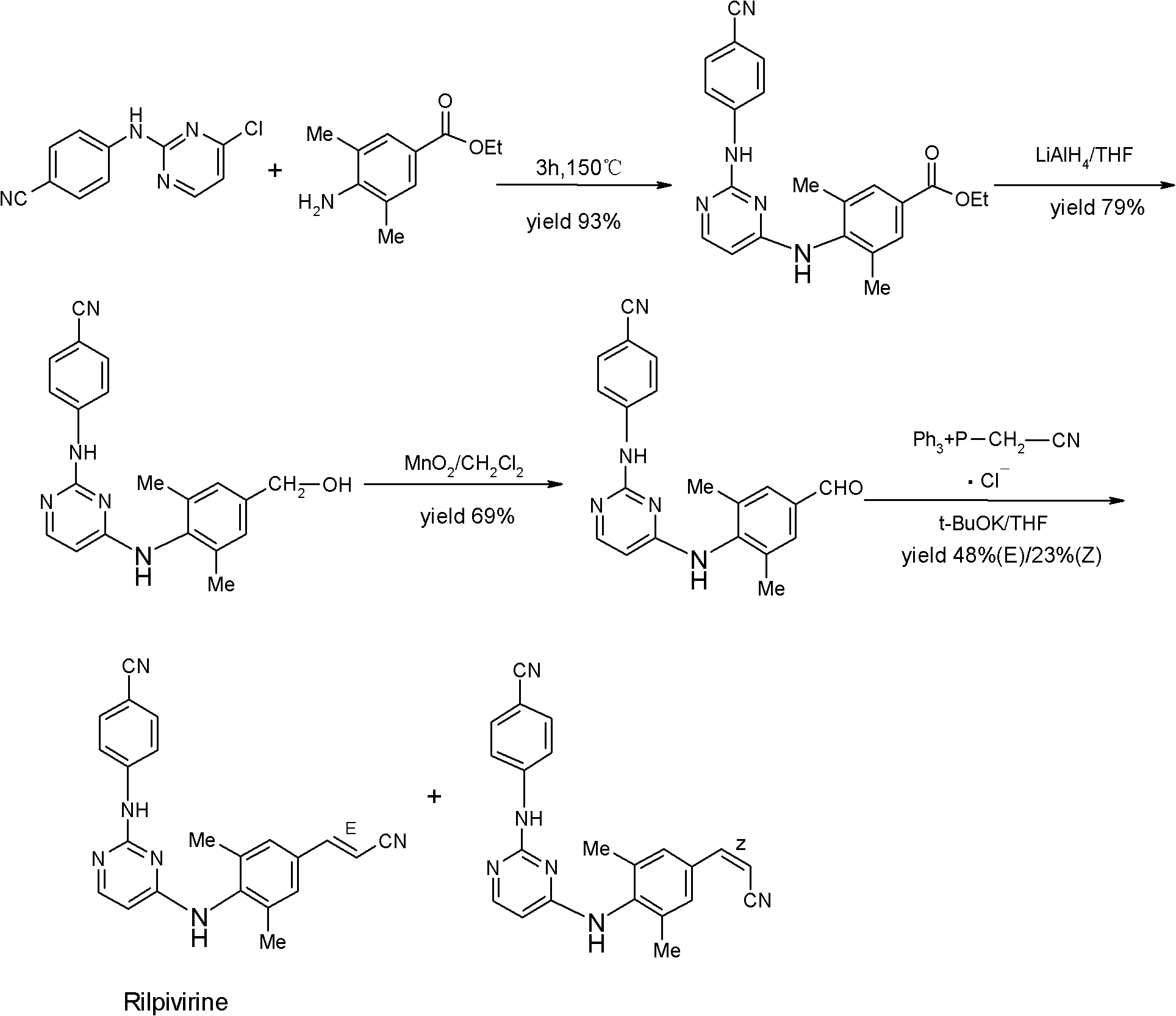
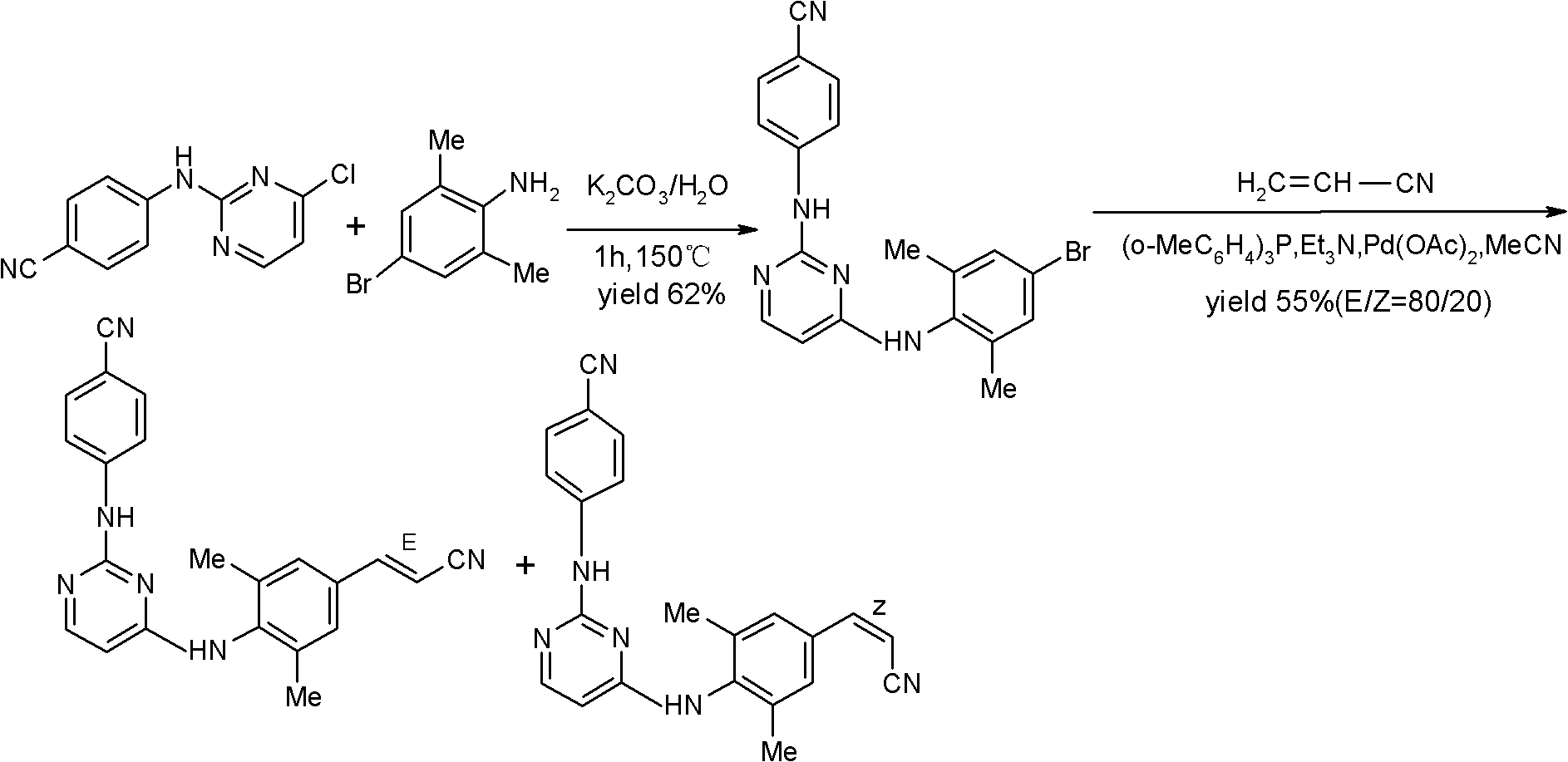
Rilpivirine intermediate and preparation method and application thereof
A technology of rilpivirine and intermediates, applied in the field of drug synthesis, can solve the problems of not being suitable for industrial production requirements, unavailable prices of raw materials, high total cost, etc., to meet the needs of large-scale industrial production, simple preparation method and low cost synthetic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: preparation intermediate of the present invention
[0051]
[0052] Step A: Synthesis of 3-(4-amino-3,5-dimethyl-phenyl)-acrylonitrile III
[0053] Under the protection of argon, dissolve 20.0g (0.10mol) of 4-bromo-2,6-dimethyl-aniline II in 100ml of N,N-dimethylformamide (DMF), stir to dissolve, add sodium iodide 0.75 g (5mmol), after heating to 80°C for 0.5 hours, add sodium acetate 9.84g (0.12mol), 1.1g palladium carbon (Pd / C, 10%), acrylonitrile 6.36g (0.12mol) successively, and heat to React at 140°C for 8 hours; cool to room temperature, filter, concentrate the filtrate to dryness, add 200ml ethyl acetate to dissolve, wash twice with saturated brine, dry over anhydrous magnesium sulfate, concentrate to dryness, and recrystallize from acetonitrile to obtain: 3- (4-Amino-3,5-dimethyl-phenyl)-acrylonitrile III 14.74g (0.0857mol), the molar yield is 85.7%; 1 HNMR (DMSO) δ: 2.35 (6H, s), 4.0 (2H, s), 5.88 (1H, d), 6.66 (2H, s), 7.38 (1H, d).
[0054...
Embodiment 2
[0060] Embodiment 2: preparation intermediate of the present invention
[0061]
[0062] Step C: Synthesis of 3-(4-amino-3,5-dimethyl-phenyl)-acrylamide V
[0063] Under the protection of argon, dissolve 20.0g (0.100mol) of 4-bromo-2,6-dimethyl-aniline II in 100ml of acetonitrile, stir to dissolve; add 0.75g (0.005mol) of sodium iodide, and heat to reflux; reflux After reacting for 0.5 hours, 2.24 g (0.010 mol) of palladium acetate, 6.09 g (0.020 mol) of tris(2-methylphenyl) phosphorus, 6.36 g (0.12 mol) of acrylamide and 14.6 g (0.200 mol) of ethylenediamine were added successively. mol), heated to reflux; after 8 hours of reflux reaction, cooled to room temperature, added 500ml of ethyl acetate to dissolve, washed once with saturated sodium bicarbonate solution and saturated brine, dried over anhydrous magnesium sulfate, concentrated to dryness, and distilled with isopropyl Ether recrystallized to obtain: 3-(4-amino-3,5-dimethyl-phenyl)-acrylamide V 15.9 g (0.0837 mol), ...
Embodiment 3
[0068] Embodiment 3: preparation intermediate of the present invention
[0069]
[0070] Step C: the same as that described in step C in Example 2.
[0071] Step F: Synthesis of 3-(4-amino-3,5-dimethyl-phenyl)-acrylonitrile III
[0072] Under the protection of argon, 9.5 g (0.050 mol) of (4-amino-3,5-dimethyl-phenyl)-acrylamide V was added into 100 ml of acetonitrile, cooled to 0°C, and 38.33 g of phosphorus oxychloride was added dropwise ( 0.250mol); after dropping, warm up to room temperature, stir for 2 hours, then heat to reflux; after reflux reaction for 3 hours, concentrate under reduced pressure to dryness; add 50ml of water and 100ml of ethyl acetate to dissolve, adjust the reaction solution to Neutral, liquid separation; the organic phase was washed with saturated sodium bicarbonate solution and saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure to dry crude product 9.7g, recrystallized from acetonitrile to obtain: 3-(4-am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com