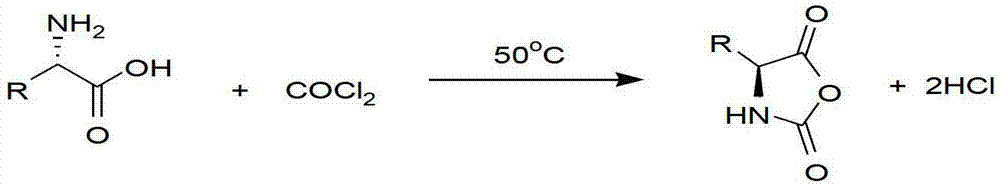
Method for preparing amino acid N-carboxyanhydride
A technique for intracyclic acid anhydride and amino acid, applied in the field of preparing amino acid N-carboxy intracyclic acid anhydride, can solve the problems of reduced yield, low yield and the like, and achieve the effects of improving yield, improving decomposition rate and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] The present invention will be illustrated in more detail by the following examples, but these examples are for illustrative purposes only and are not intended to limit the scope of the present invention.
Embodiment 1
[0029]Put 25 grams of L-alanine, 0.5 grams of activated carbon and 450 milliliters of anhydrous tetrahydrofuran into the above-mentioned reaction device. At 50 ° C, add dropwise 50 milliliters of anhydrous tetrahydrofuran solution containing 33.4 grams of triphosgene. After the addition is complete, After stirring at this temperature for 3 hours, the system was almost clear. The reaction mixture was filtered with celite filter aid. The filtrate was concentrated to one-fifth of the original volume, 2 liters of petroleum ether was added, and the crude product was obtained by filtration. Recrystallization from a mixed solvent of ethyl acetate and petroleum ether gave 21 g of a white solid, melting point: 91-92°C.
Embodiment 2
[0031] Put 25 grams of L-alanine, 0.25 grams of activated carbon and 450 milliliters of anhydrous tetrahydrofuran into the above-mentioned reaction device. At 50° C., add dropwise 50 milliliters of anhydrous tetrahydrofuran solution containing 27.8 grams of triphosgene. After the addition is complete, After stirring at this temperature for 5 hours, the system was almost clear. The reaction mixture was filtered with celite filter aid. The filtrate was concentrated to one-third of the original volume, 250 ml of petroleum ether was added, and the crude product was obtained by filtration. Recrystallization from a mixed solvent of ethyl acetate and petroleum ether gave 7 g of a white solid, melting point: 91-92°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com