Epinephelus coioides interferon IFNgamma2 and preparation method and application thereof
A technology of slanted grouper and interferon, applied in the field of interferon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Cloning of the grouper interferon IFNγ2
[0040] 1. Extraction of total RNA from head and kidney of grouper
[0041] Take healthy whole grouper fish, anesthetize them in ice bath for about 2 minutes, kill the fish and take samples, separate the head and kidney tissue, and extract the head and kidney total RNA of grouper with Trizol reagent, and its OD260 / 280 = 1.91.
[0042] 2. Synthesis of cDNA First Strand
[0043] Take 1 μg of the total RNA sample of grouper head kidney and perform DNase treatment to remove the contamination of genomic DNA, mix it with RNA Oligo dT (sequence shown in SEQ ID NO:3), perform reverse transcription, and place the obtained product in- Store at 20°C for later use.
[0044] 3. Cloning of the complete cDNA sequence of the IFNγ2 gene of the grouper
[0045] According to the information in the grouper genome database, design specific primers at both ends of the spliced sequence of the IFNγ open reading frame, the upstream prime...
Embodiment 2
[0046] Example 2. Expression of the recombinant protein of interferon IFNγ2 from the grouper
[0047] 1. Construction of recombinant expression plasmids
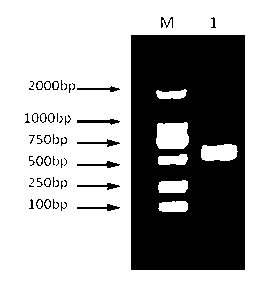
[0048] Using the pTZ57R / T plasmid containing the IFNγ2 coding gene as a template, design a pair of upstream primers SEQ ID NO:6 containing EcoRI restriction sites and downstream primers SEQ ID NO:7 containing XhoI restriction sites, and amplify by PCR A single band with a product size of about 550 bp was obtained, and the electrophoresis results were as follows figure 2 As shown, wherein, M is a DNA Marker, and 1 is an IFNγ2 nucleotide target fragment with a restriction site. The mature peptide coding sequence of grouper interferon IFNγ2 was cloned into the prokaryotic expression vector pET-22b to construct the recombinant expression vector pET22b-IFNγ2, (the construction process is as follows image 3 shown). The exogenous gene sequence in the expression vector was identified by sequencing.
[0049] 2. Prokaryotic expr...
Embodiment 3
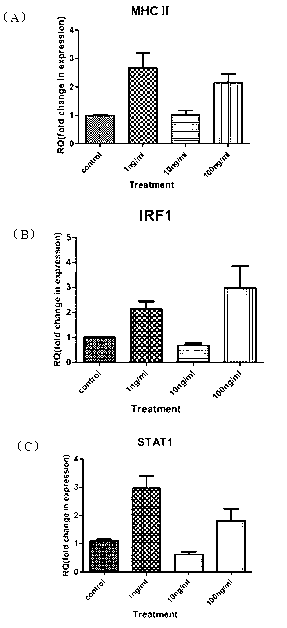
[0054] Example 3. Activity analysis of the influence of the recombinant protein of the grouper interferon IFNγ2 obtained in Example 2 on the expression of immune-related genes
[0055] Sampling of rapidly frozen and anesthetized experimental fish, cut head and kidney tissue, soaked in appropriate amount of RPMI1640 medium, and placed on ice; put the head and kidney tissue in an ultra-clean workbench and shredded slightly, after removing the connective tissue, Grind with the frosted surface of the baked frosted glass slide until it is fine; pass the ground cells with the medium through the Cell strainer (BD Falcon, 70 μm, Nylon) and transfer to a 50ml centrifuge tube: Centrifuge, discard the supernatant Add 2 ml of RPMI 1640 medium to resuspend the cells, wash, centrifuge, and resuspend the cells with 2 ml of complete medium (RPMI 1640 containing 2 mM L-glutamine, 10% FBS and 1% penicillin / streptomycin) to adjust the number of cells to 1 x 10 7 / mL, add 2 mL of the above cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com