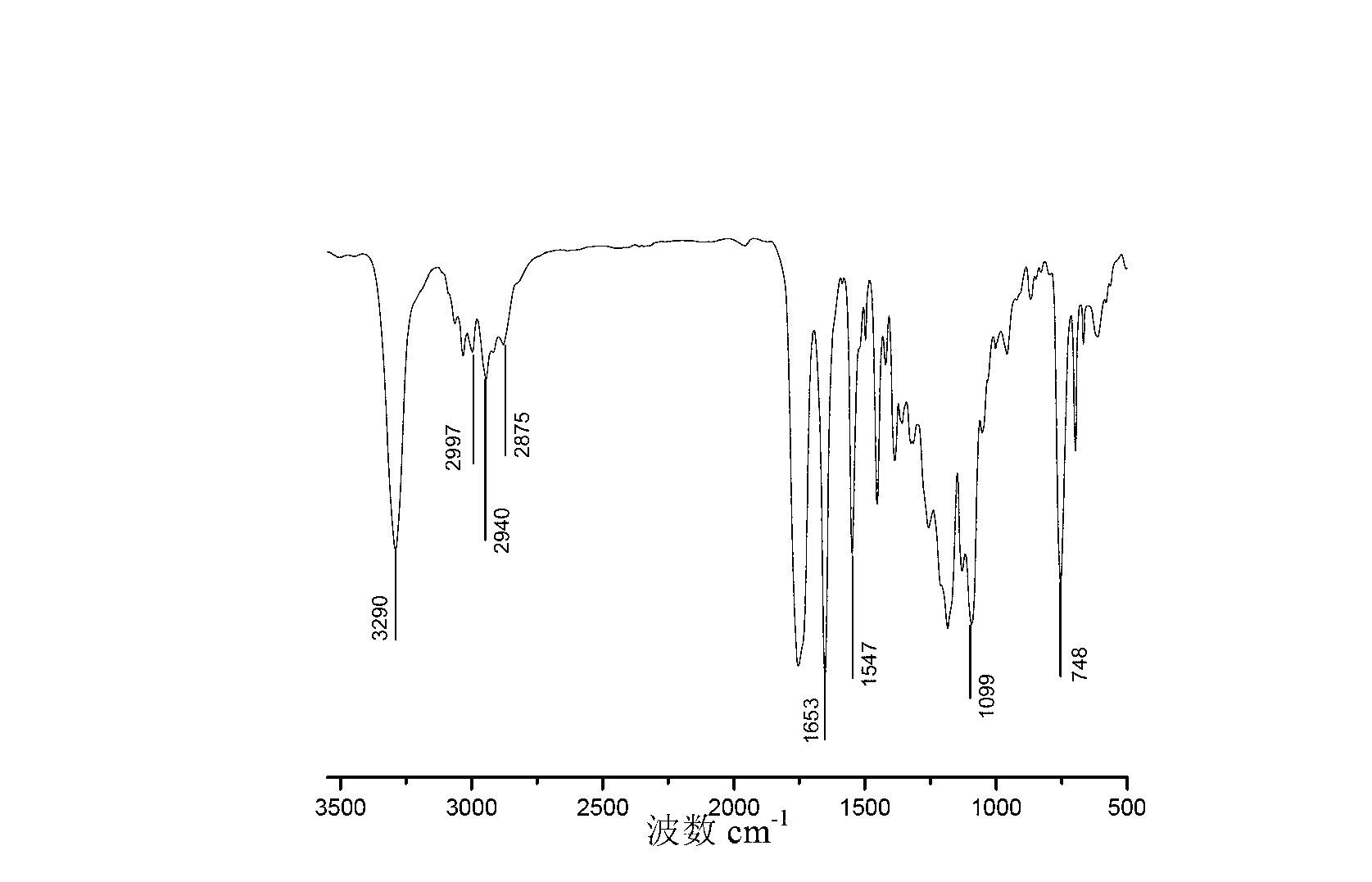
Preparation method of polylactic acid glycollic acid-polypeptide-polyethylene glycol diblock-grafted copolymer
A technology of poly(lactic-co-glycolic acid) and di-block copolymer, which is applied in the field of biodegradable polymer material preparation, and achieves the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, adopt the following steps:
[0022] 1) Synthesis of Polypeptide Homopolymers Containing Terminal-NCO Groups:
[0023] Add 15 grams of poly(γ-benzyl-L-glutamate) with a molecular weight of 10,000, 4 grams of 2,4-toluene diisocyanate and 250 ml of dimethyl sulfoxide solvent into the dry reactor, and add the above Dibutyltin dilaurate with a total reactant weight of 3‰, under an inert atmosphere, stirred and reacted at 50° C. for 40 minutes, terminated the reaction, removed excess diisocyanate by dialysis to obtain the target object, and the molecular weight of the target object was 10174;
[0024] 2) Synthesis of poly(lactic-co-glycolic acid-polypeptide diblock copolymer):
[0025] Add 10 grams of poly(γ-benzyl-L-glutamate) containing terminal -NCO groups, 30 grams of polylactic acid with a molecular weight of 2000 and a ratio of lactic acid and glycolic acid of 75 / 25 to the dry reactor Glycolic acid monolauryl ether and 300ml dimethyl sulfoxide solvent, a...
Embodiment 2
[0031] 1) Synthesis of Polypeptide Homopolymers Containing Terminal-NCO Groups:
[0032] Add 14 grams of poly(γ-ethyl-L-glutamate) with a molecular weight of 15,000, 3.2 grams of 2,4-toluene diisocyanate and 200 ml of dimethyl sulfoxide solvent into the dry reactor, and add the above reaction Dibutyltin dilaurate with a total weight of 4‰, under an inert atmosphere, stirred and reacted at 52° C. for 50 minutes, terminated the reaction, removed excess diisocyanate by dialysis to obtain the target object, and the molecular weight of the target object was 15174;
[0033] 2) Synthesis of poly(lactic-co-glycolic acid-polypeptide diblock copolymer):
[0034] Add 9 grams of poly(γ-ethyl-L-glutamate) containing terminal -NCO groups, 29.5 grams of poly(lactic acid ethanol) with a molecular weight of 2500 and a ratio of lactic acid and glycolic acid of 75 / 25 to the dry reactor Acid monododecyl ether and 290ml of 1,1,2-trichloroethane solvent, then add dibutyltin dilaurate with a total ...
Embodiment 3
[0039]1) Synthesis of Polypeptide Homopolymers Containing Terminal-NCO Groups:
[0040] Add 12 grams of poly(γ-methyl-L-glutamate) with a molecular weight of 20,000, 2.5 grams of 2,4-toluene diisocyanate and 190 ml of dimethyl sulfoxide solvent into a dry reactor, and add the above reaction Dibutyltin dilaurate of total weight 5‰, under an inert atmosphere, stirred and reacted at 55° C. for 60 minutes, terminated the reaction, removed excess diisocyanate by dialysis to obtain the target object, and the molecular weight of the target object was 20174;
[0041] 2) Synthesis of poly(lactic-co-glycolic acid-polypeptide diblock copolymer):
[0042] Add 8 grams of poly(γ-methyl-L-glutamate) containing terminal -NCO groups, 29.1 grams of poly(lactic acid ethanol) with a molecular weight of 3000 and a ratio of lactic acid and glycolic acid of 75 / 25 to the dry reactor Acid monododecyl ether and 280ml dimethyl sulfoxide solvent, then add dibutyltin dilaurate with a total weight of 5‰ o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com