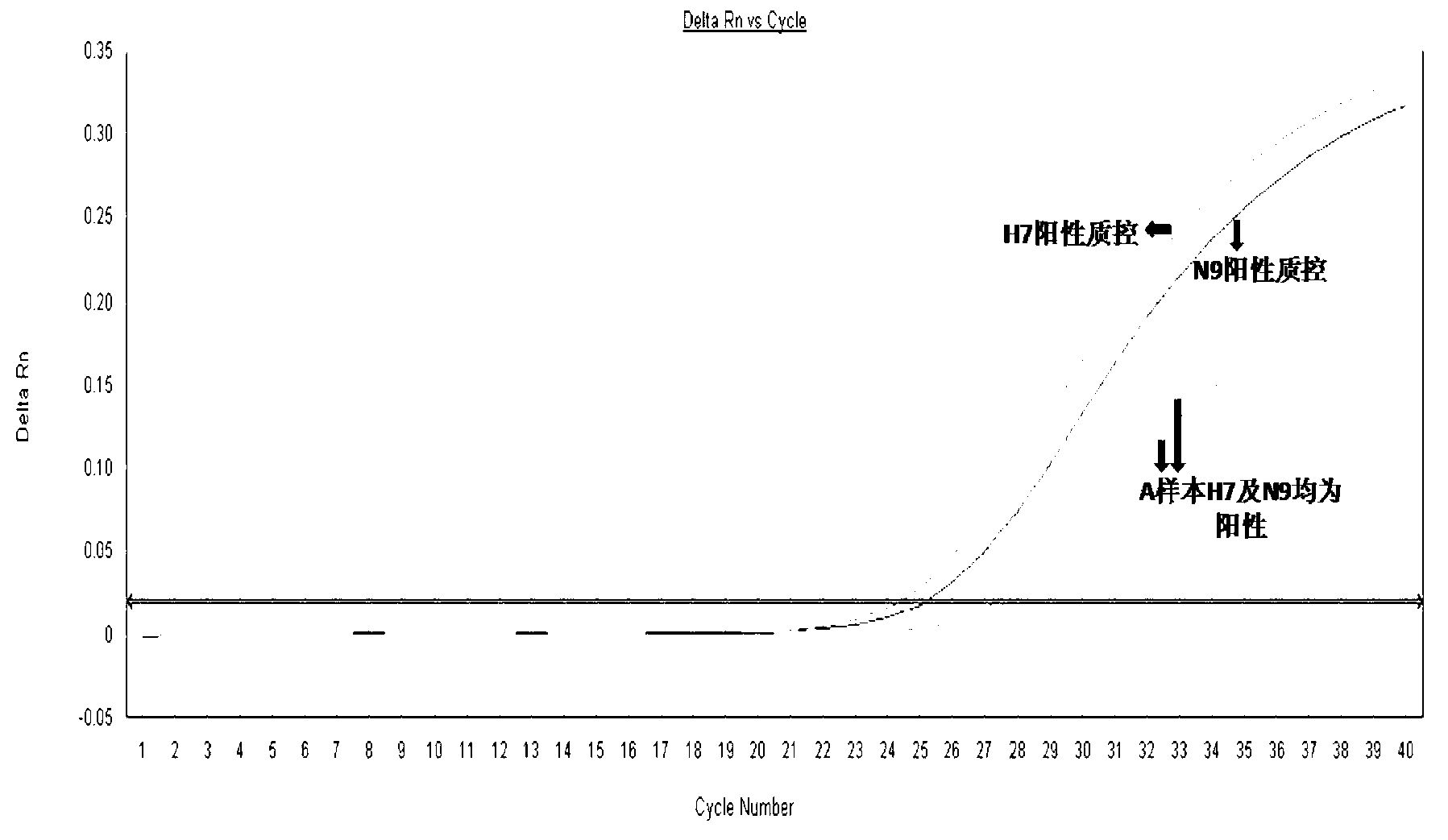
Kit for testing H7N9 bird flu virus using real time fluorescence quantitative PCR
A real-time fluorescence quantitative and avian influenza virus technology, applied in the field of life sciences and biology, to achieve high accuracy, reduce the fatality rate, control the epidemic and gain time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
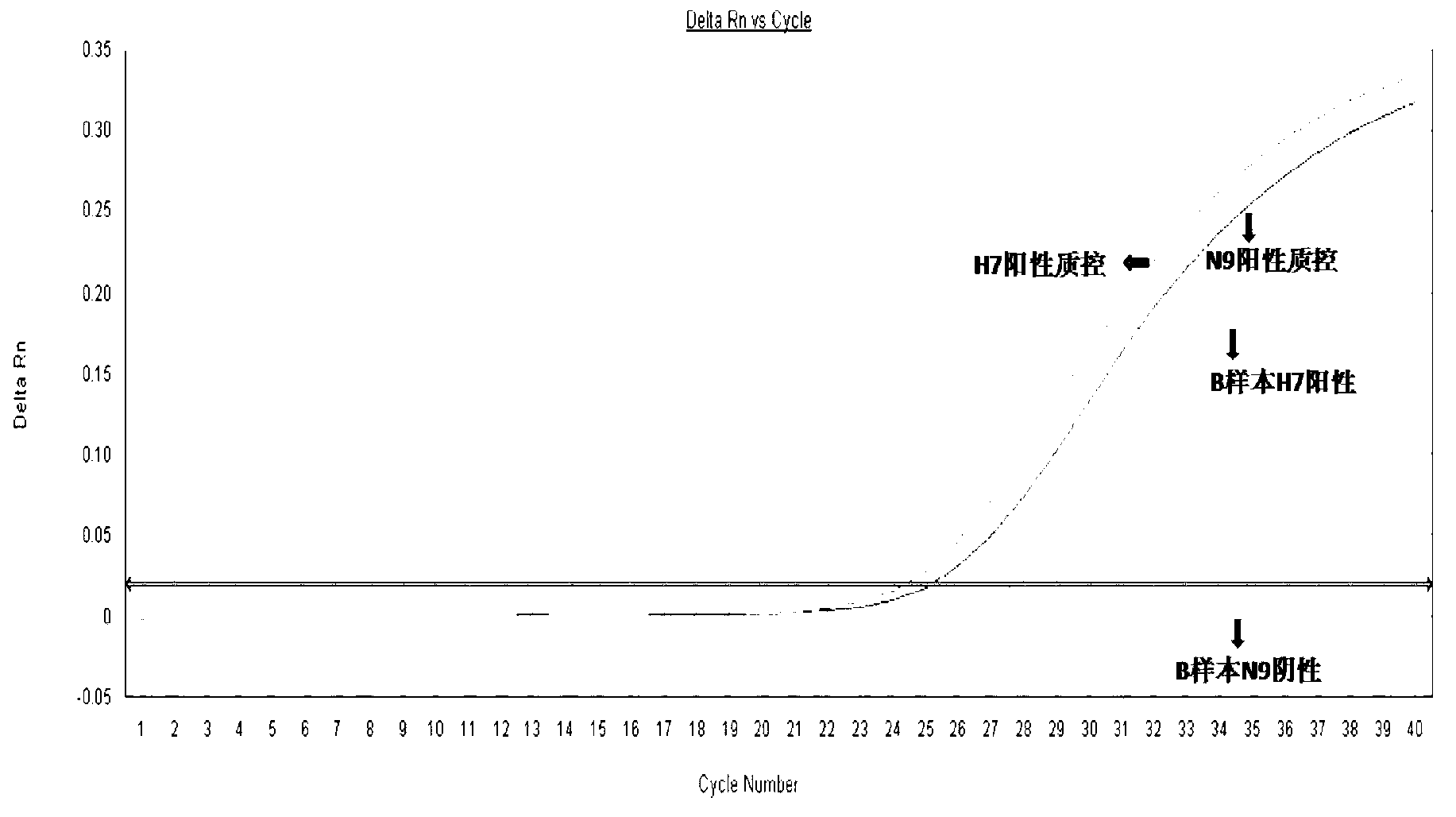
Image
Examples
Embodiment 1
[0046] A kit for detecting H7N9 avian influenza virus nucleic acid, including:
[0047] (i) RNA extraction reagent: QIAamp Viral RNA mini Kit;
[0048] (ii) Reagent for reverse transcription: 5×RT-Buffer, 100uM Primer Mix, 100U / ul ReverTra Ace reverse transcriptase, DEPC water;
[0049] (iii) Real-time fluorescence quantitative PCR reagents: qPCR Mix, 50×ROX, 2 pairs of primers and corresponding probes for amplifying sample cDNA, sterile water, of which:
[0050] Primers and probes for detecting H7N9 avian influenza virus envelope surface hemagglutinin H7:
[0051] H7-F: GGGWTTCACMTACAGYGGAATAAG
[0052] H7-R: ACAGGARCCATTTCATCTCTCYGC
[0053] H7-probe: FAM-CAACSAGTGCATGTAGGAGATCAGGWTCTTC-TAMARA
[0054] Primers and probes for detecting H7N9 avian influenza virus neuraminidase N9:
[0055] N9-F: TGARTGCAGGTTCTATGCTCTCA
[0056] N9-R: TGTATACTGTGGGYGGTGATGA
[0057] N9-Probe: HEX-CTCAAACGGAACAATACACGATAGGTCCCA-TAMARA
[0058] The using method of described kit comprises ...
Embodiment 2
[0063] Embodiment 2: detection method
[0064] 1. Extraction of RNA from samples to be tested
[0065] 1) Pipette 560ul of prepared buffer AVL (containing carrier RNA) into a 1.5ml centrifuge tube. (Adjust the buffer AVL-carrier RNA proportionally according to the actual amount of the sample).
[0066] 2) Add 140ul plasma, serum, urine, cultured cell supernatant or cell-free body fluid to the centrifuge tube containing buffer AVL-carrier RNA. Mediate for 15 seconds and mix well. (To ensure lysis efficiency, it must be mixed thoroughly to form a homogeneous solution. Samples that have been dissolved only once can still be used).
[0067] 3) Leave at room temperature for 10 minutes. (10 minutes is enough, prolonging the time will not increase the quality of the product. Buffer AVL can inactivate potential contamination and RNase).
[0068] 4) Centrifuge briefly, and throw the liquid on the cap back to the bottom of the tube.
[0069] 5) Add 560ul absolute ethanol (96%-10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com