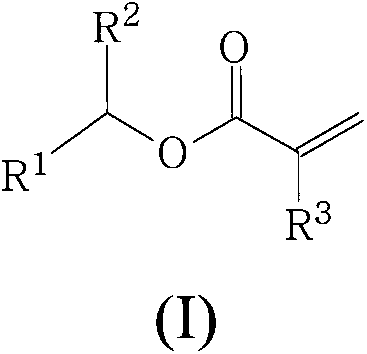
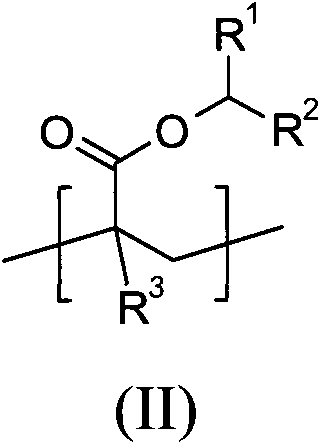
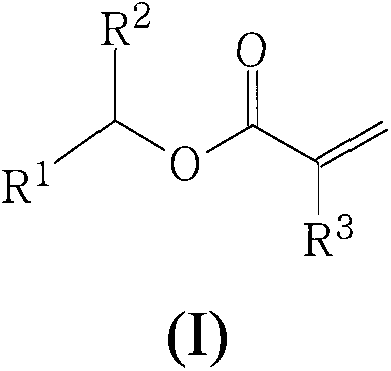
Polymers derived from secondary alkyl (meth)acrylates
An acrylate, polymer technology for use in the field of polymers derived from secondary alkyl (meth)acrylates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0271] Objects and advantages of this invention are further illustrated by the following examples, but the particular materials and amounts thereof recited in these examples, as well as other conditions and details, should not be construed to unduly limit this invention. Unless otherwise noted, materials used in the following examples were purchased from Alfa Aesar (Ward Hill, MA) or Sigma-Aldrich Company, St. Louis, MO. (St. Louis, MO)).
[0272] Test Method 1: Differential Scanning Calorimetry (DSC) Analysis of Monomer and Homopolymer Films
[0273] Approximately 10 mg of a given monomer was placed on a separate standard aluminum DSC pan (Thermal Analysis T080715), which was then placed in a Differential Scanning Calorimeter (DSC, TA Instruments, New Castle, Delaware). DE)) in the autosampler. For the analysis of each sample, the disc was placed individually on one of the differential posts in the closed cell of the DSC and an empty reference disc was placed on the oppos...
example 1
[0285] Example 1: Octyl Acrylate Isomer Blend from 1-Octene
[0286] To 200.0 grams (g) (1.782 moles, 1 equivalent (eq)) of 1-octene at 50°C was added 145.1 g (2.014 moles, 1.13 eq) of acrylic acid (Alfa Aesar) and 5.349 g (0.03564 mol, 0.02 eq) solution of trifluoromethanesulfonic acid (Alfa Aesar). After holding at 50°C for 10 minutes, the mixture was heated to 70°C with an exotherm to 90°C. The mixture was cooled to 70°C within one hour and kept at 70°C for 15 hours. 411.3 g of a 9.1% by weight sodium bicarbonate solution (saturated aqueous solution) (0.4456 mol, 0.25 eq) were added to the reaction mixture. The mixture was stirred for 30 minutes, then allowed to separate the phases. Further, 411.3 g of a 9.1% by weight sodium bicarbonate solution (saturated aqueous solution) (0.4456 mol, 0.25 eq) was added thereto and stirred for 30 minutes. 300 mg phenothiazine and 100 mg (methoxyhydroquinone) (MEHQ) (Alfa Aesar) were added to the organic phase and the mixture was di...
example 2
[0287] Example 2: Octyl Acrylate Prepared Using Excess Acrylic Acid
[0288] To 10.0 g (0.0891 moles, 1 eq) of 1-octene and 0.00679 g of copper(II) chloride dihydrate were added 19.2 g (0.267 moles, 3 eq) of acrylic acid and 0.802 g (0.00534 moles, 0.06 eq) of triflic acid mixture. The mixture was heated to 40°C for 30 minutes, then to 70°C for 15 hours. The reaction gave an 83% conversion of the desired acrylate based on the 1-octene consumed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com