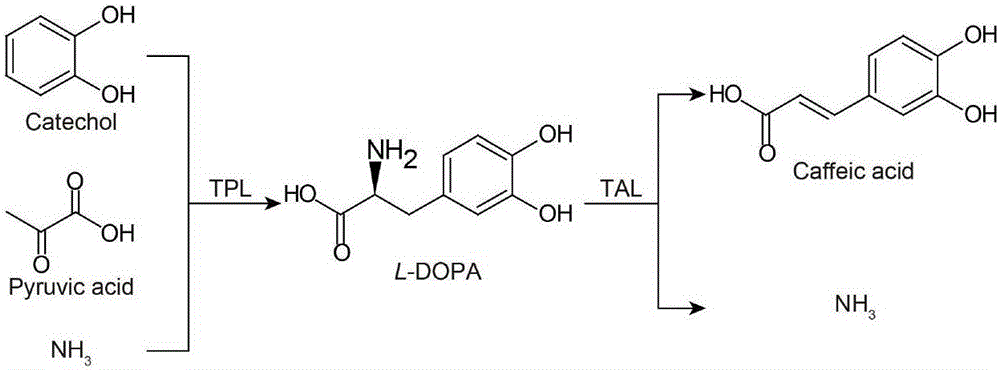
Efficient biosynthesis method for transforming low-value compounds into caffeic acid
A technology of biosynthesis and caffeic acid, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of no significant increase in yield, unsatisfactory effect, and low substrate conversion rate of caffeic acid production, reaching Effects of cheap mass production and easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Construction method of recombinant Escherichia coli
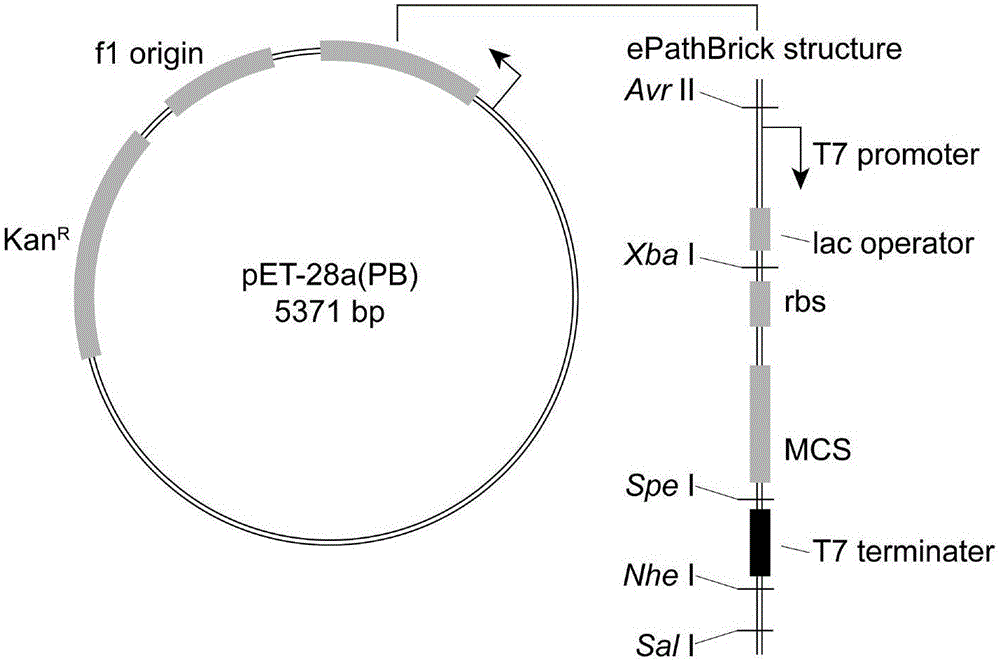
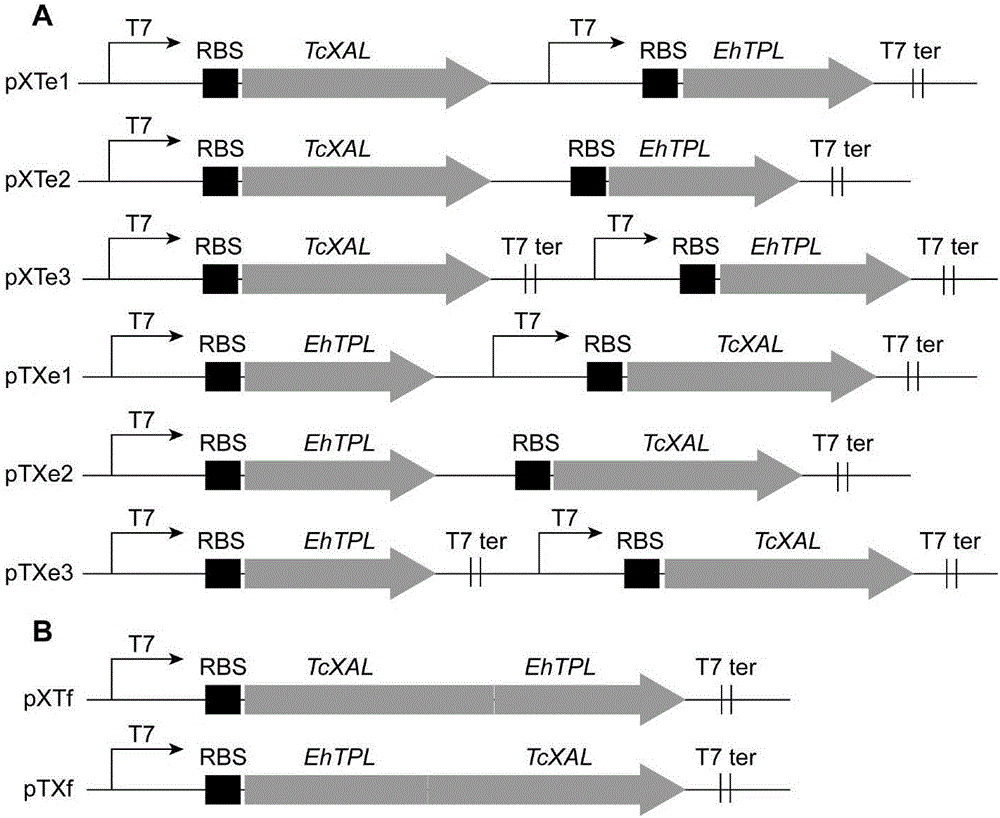
[0032] The genes EhTPL and TcXAL were optimized and synthesized by GenScript Biotechnology Co., Ltd., and cloned into pUC57-Simple. The recombinant plasmids were named pUC57-EhTPL and pUC57-TcXAL, respectively. pET28a(PB) is an ePathBrick expression vector constructed on the basis of pET-28a(+). The vector contains homologous enzymes Avr II, Xba I, Spe I and Nhe I, namely the downstream Sal I. Different ePathBrick strategies can be used to construct Co-expression constructs. The structure of pET28a(PB) is as follows figure 2, the DNA sequence is SEQ ID NO.5. The recombinant vectors pUC57-EhTPL, pUC57-TcXAL and the expression vector pET28a(PB) were digested with restriction endonuclease Bam HI / Hind III respectively, the uncut products were separated by agarose gel electrophoresis, and the target gene EhTPL (1371bp) was recovered respectively , TcXAL (2070bp) and expression vector pET28a(PB) (5371bp). Ac...
Embodiment 2
[0037] Example 2 Analysis of recombinant Escherichia coli strCL-1 catalyzing the synthesis ability of levodopa
[0038] In order to verify the ability of recombinant escherichia coli E.coli strCL-1 in Table 1 to catalyze the synthesis of levodopa from catechol, the transformation method described in Example 1 was used to transform the escherichia coli BL21 ( DE3) As a blank control, PBS (50mM, pH 7.0) was used as the reaction medium, reacted at 37°C and 220rpm, samples were taken at specific time points, and the synthesis of levodopa was determined.
[0039] The results showed that levodopa was synthesized in the reaction system catalyzed by E.coli strCL-1, but levodopa was not detected in the blank control. This result verified the ability of EhTPL to catalyze the synthesis of levodopa from catechol, and the synthesis process could not proceed spontaneously in the absence of tyrosine benzene lyase. The chromatogram and mass spectrum of levodopa are as follows Figure 4 , wh...
Embodiment 3
[0040] Example 3 Recombinant Escherichia coli catalyzes the ability to synthesize caffeic acid from catechol
[0041] In order to verify the ability of the recombinant Escherichia coli listed in Table 1 to transform catechol, sodium pyruvate and ammonium chloride into caffeic acid, the collected bacteria were washed with 25 mL of PBS, centrifuged and resuspended in an equal volume of PBS (50 mM, pH 7.0), the cell concentration is OD 600 =18±1. Simultaneously add 0.65M NH 4 Cl, 50 mM sodium pyruvate and 50 mM catechol were used as substrates for the reaction, and the reaction was carried out on a constant temperature shaker at 37° C. and 220 rpm. Escherichia coli BL21(DE3) containing empty plasmid pET28a(PB) was used as blank control. Samples were taken at specific time points to determine the synthesis of caffeic acid. Take the maximum production of caffeic acid in 0-10 hours as the corresponding maximum production of the strain.
[0042] The results showed that the recom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com