Method for improving structural isomerism catalytic activity of CE enzyme and mutant thereof
A mutant and epimerase technology, applied in the field of enzyme engineering, can solve the problem of poor substrate affinity, which restricts the process of industrial application, and the catalytic properties of substrate affinity, lactulose conversion rate and thermal stability have not been greatly improved and other issues to achieve good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Determining the substrate binding site of CsCE and constructing a mutant plasmid
[0040] (1) Comparative analysis of amino acid sequence and crystal structure to determine the mutation site
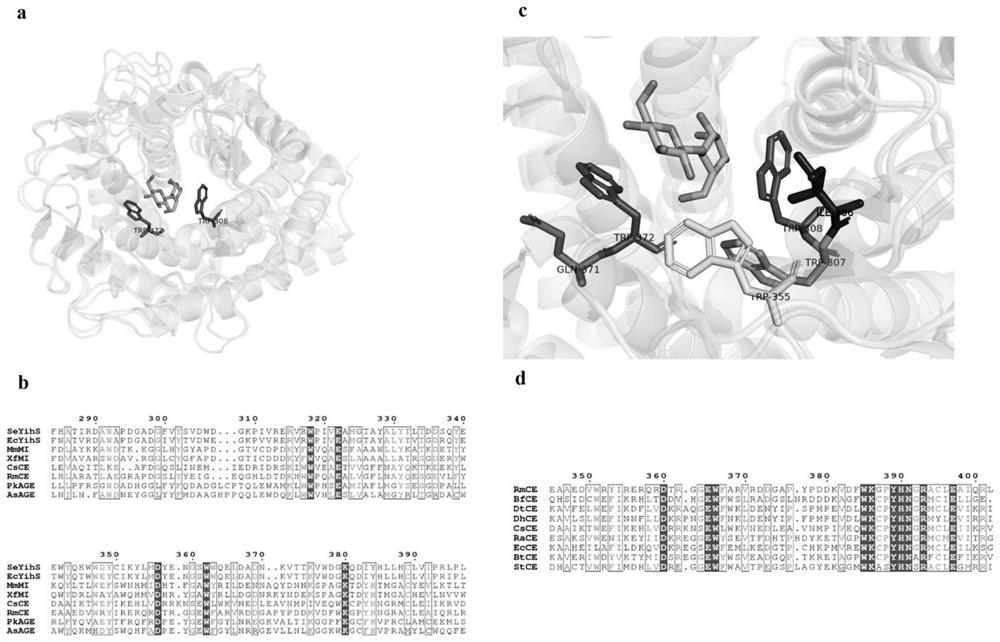
[0041] like figure 1 As shown in the crystal structure comparison diagram and amino acid sequence comparison, the two tryptophan (Tryptophan, W) in the active center of CE enzyme can recognize and fix the disaccharide substrate, and the tryptophan W308 near the reducing end of the substrate is in the N - The acetyl-glucosamine superfamily is conserved, while the non-reducing end tryptophan W372 is only conserved in the CE enzyme family. Therefore, it is speculated that the W372 site affects the activity of CE enzymes in catalyzing disaccharides.
[0042] (2) Construction of wild-type recombinant plasmid pET-28b-CsCE
[0043] ① The whole genome of Caldicellulosiruptorsaccharolyticus DSM 8903 was extracted as a template, primers were designed according to the CsCE gene s...
Embodiment 2
[0071] Example 2: Remodeling the substrate binding pocket and constructing mutants based on semi-rational design
[0072] In order to improve the structural isomerization activity of CsCE, a semi-rational design strategy was used to perform saturation mutations on the amino acids around the substrate-binding related sites CsCE-W308 and CsCE-W372. Sequence alignment and crystal structure analysis of CE family enzymes were carried out, and two residues near tryptophan and at the entrance of the substrate were selected for molecular modification ( figure 2 ), respectively CsCE-I306 and CsCE-W307 near the substrate reducing end CsCE-W308 (divided into A region); CsCE-Q371 near the substrate non-reducing end CsCE-W372 (divided into B region); CsCE active center At the entrance of CsCE-W355 (divided into C region), the PCR method was used to perform saturation mutation on these 4 sites.
[0073] The PCR amplification reaction system and reaction amplification conditions were the s...
Embodiment 3
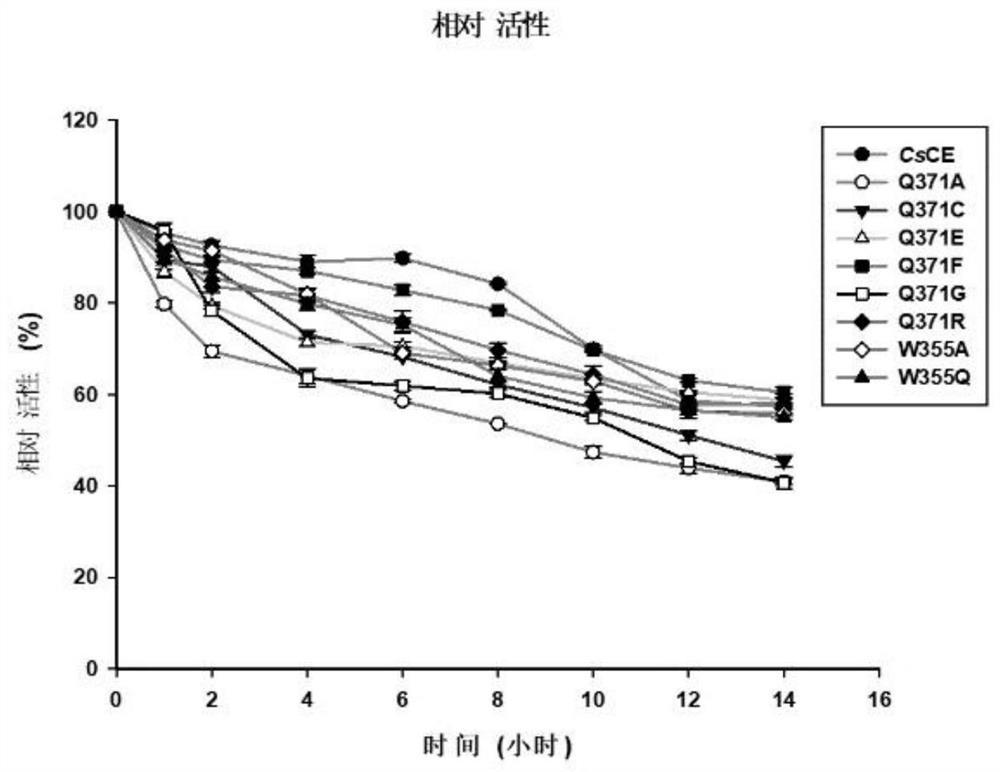
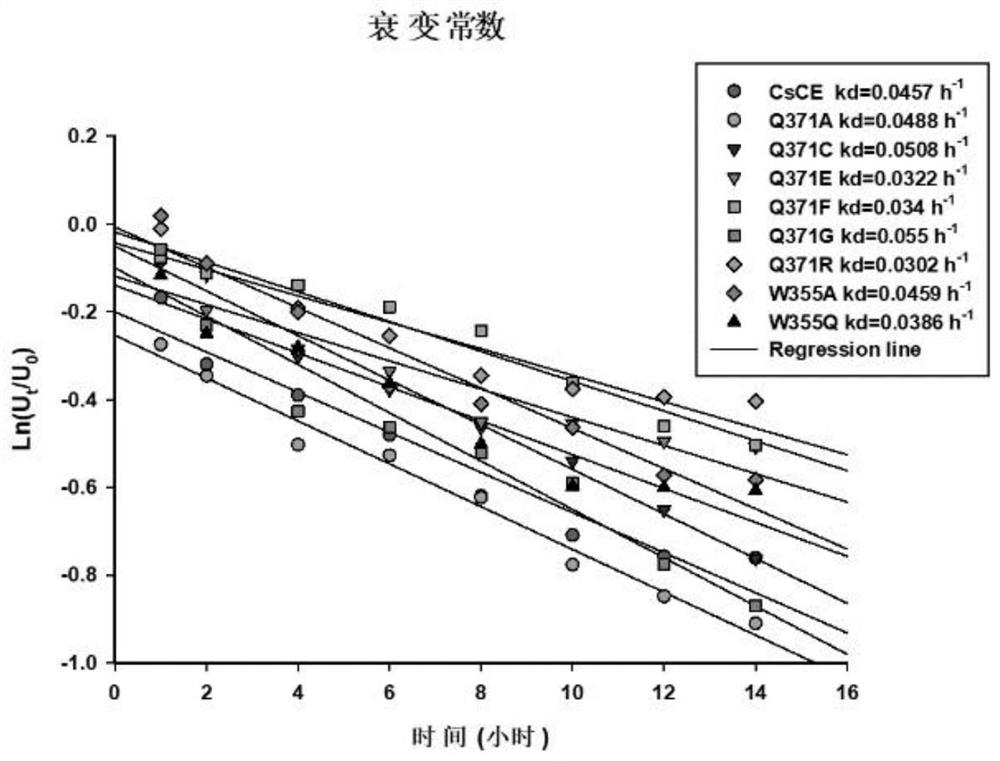
[0077] Example 3: Study on Enzymatic Properties of Cellobiose Epimerase
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com