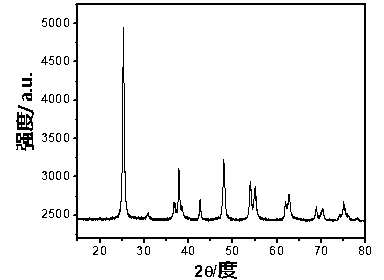
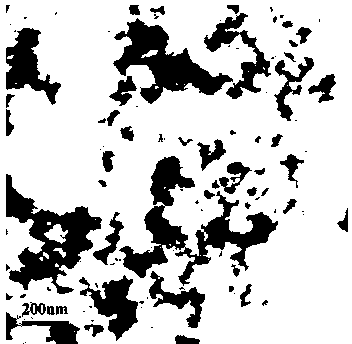
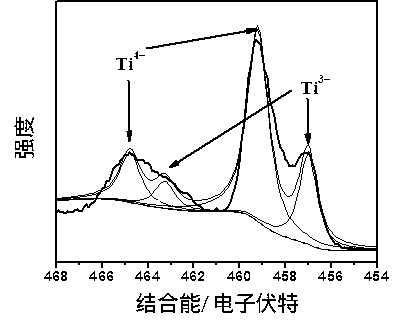
Preparation method of self-doped titanium dioxide nanorod
A technology of titanium dioxide and nanorods, which is applied in the field of preparation of self-doped titanium dioxide nanorods, to avoid the influence of shape and stability, high visible light photocatalytic activity, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: In a 250 ml beaker, 4 ml of hydrazine (N 2 H 4 ?H 2 O, 80%) and 62 ml ethylene glycol (C 2 H 6 O 2 ), magnetically stirred for 10 min, and then slowly add 4 ml of butyl titanate (Ti(OC 4 H 9 ) 4 ), that is, the volume ratio of butyl titanate:hydrazine:ethylene glycol is 1:1:15.5, stirred vigorously for 30 minutes, and then transferred to a 100ml hydrothermal reactor lined with tetrafluoroethylene and placed in a constant temperature of 200 ℃ In the dry box, keep the reaction for 24 h under solvothermal conditions. After the reaction, it was naturally cooled to room temperature, washed with distilled water and absolute ethanol three times each, and the product was placed in a 70 ℃ drying box for vacuum drying for 24 hours. The titanium dioxide nanorods prepared by this step are about 70-80 nm long and 10-15 nm wide.
Embodiment 2
[0029] Example 2: In a 250 ml beaker, 6 ml of hydrazine (N 2 H 4 ?H 2 O, 80%) and 60 ml ethylene glycol (C 2 H 6 O 2 ), magnetically stirred for 10 min, and then slowly add 4 ml of butyl titanate (Ti(OC 4 H 9 ) 4 ), that is, the volume ratio of butyl titanate:hydrazine:ethylene glycol is 1:1.5:15, stirred vigorously for 60 minutes, and then transferred to a 100 ml hydrothermal reactor lined with tetrafluoroethylene and placed at 200 ℃ In a constant temperature drying oven, keep the reaction for 24 h under solvothermal conditions. After the reaction is over, cool to room temperature naturally, wash with distilled water and absolute ethanol three times each, and place the product in a drying oven at 80 ℃ for vacuum drying for 12 h. The titanium dioxide nanorods prepared by this step are about 70-80 nm long and 10-15 nm wide.
Embodiment 3
[0030] Example 3: Add 12 ml of hydrazine in a 250 ml beaker (N 2 H 4 ?H 2 O, 80%) and 54 ml ethylene glycol (C 2 H 6 O 2 ), magnetically stirred for 10 min, and then slowly add 4 ml of butyl titanate (Ti(OC 4 H 9 ) 4 ), the volume ratio of butyl titanate: hydrazine: ethylene glycol is 1: 3:13.5, stir vigorously for 15 minutes, then transfer to a 100 ml hydrothermal reactor lined with tetrafluoroethylene, and put it in a constant temperature of 200 ℃ In the dry box, keep the reaction for 24 h under solvothermal conditions. After the reaction, it was cooled to room temperature naturally, washed with distilled water and absolute ethanol three times each, and the product was placed in a drying oven at 60 ℃ for vacuum drying for 18 h. The titanium dioxide nanorods prepared by this step are about 60-80 nm in length and 10-15 nm in width.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com