Preparation method for high-purity magnesium oxide
A magnesia and high-purity technology, applied in the direction of magnesia, can solve the problems of large consumption of raw materials and low product purity, and achieve the effects of easy mastery, high product purity and simple production process equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
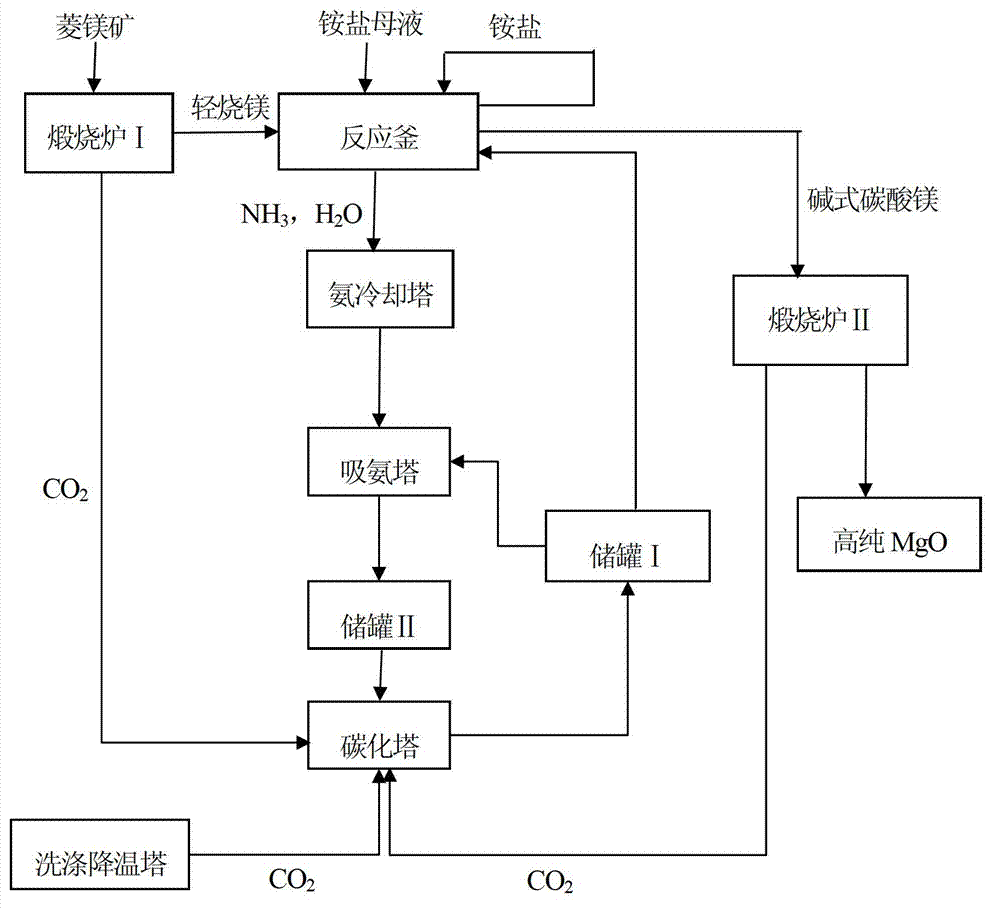
[0015] like figure 1 As shown, take 100g of magnesite, crush it to 10-50mm, and calcinate it in the calciner I at 800°C for 2 hours to generate light-burned magnesium; take 10g of light-burned magnesium, crush it to 30 mesh, and add it to 143mL The ammonium sulfate mother liquor with a fraction of 5% is heated to boiling and distilled to form a mixture of magnesium sulfate solution and ammonia gas and water. When the distillate volume is 60% of the original solution volume, stop heating, and the distilled ammonia gas and water After being cooled by the ammonia cooling tower, the mixture is absorbed by the CO from the storage tank I in the ammonia absorption tower 3 2- The carbonized ammonia water Ⅰ with a mass fraction of 3% is absorbed to form carbonized ammonia water II and enters the storage tank II, and the carbonized ammonia water II from the storage tank II is carbonized by the flue gas from the washing cooling tower in the carbonization tower to generate NH 4 + with ...
Embodiment 2
[0017] like figure 1 As shown, take 100g of magnesite, crush it to 10-50mm, and calcinate it in the calciner I at 600°C for 5 hours to generate light-burned magnesium; take 10g of light-burned magnesium, crush it to 30 mesh, and add it to 250mL In the ammonium sulfate mother liquor with a fraction of 10%, it is heated to boiling and distilled to form a mixture of magnesium sulfate solution and ammonia gas and water. When the distillate volume is 40% of the original solution volume, stop heating, and the distilled ammonia gas and water After being cooled by the ammonia cooling tower, the mixture is absorbed by the CO from the storage tank I in the ammonia absorption tower 3 2- The carbonized ammonia water Ⅰ with a mass fraction of 6% is absorbed to form carbonized ammonia water II and enters the storage tank II, and the carbonized ammonia water II from the storage tank II is absorbed by the CO from the calciner I in the carbonization tower. 2 carbonization to NH 4 + with CO...
Embodiment 3
[0019] like figure 1 As shown, take 100g of magnesite, crush it to 10-50mm, and calcinate it in the calciner I at 700°C for 4 hours to generate light-burned magnesium; take 10g of light-burned magnesium, crush it to 30 mesh, and add it to 500mL The ammonium sulfate mother liquor with a fraction of 20% is heated to boiling and distilled to form a mixture of magnesium sulfate solution and ammonia gas and water. When the distillate volume is 20% of the original solution volume, stop heating, and the distilled ammonia gas and water After being cooled by the ammonia cooling tower, the mixture is absorbed by the CO from storage tank I in the ammonia absorption tower 3 2- The carbonized ammonia water Ⅰ with a mass fraction of 8% is absorbed to form carbonized ammonia water II and enters the storage tank II, and the carbonized ammonia water II from the storage tank II is absorbed by the CO from the calciner II in the carbonization tower. 2 carbonization to NH 4 + with CO 3 2- Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com