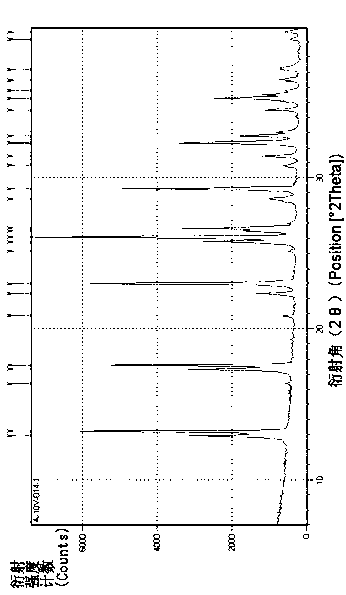
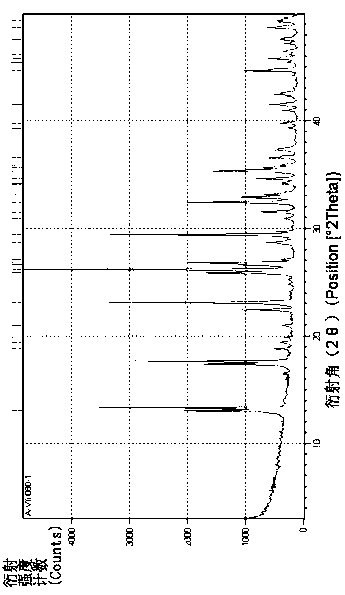
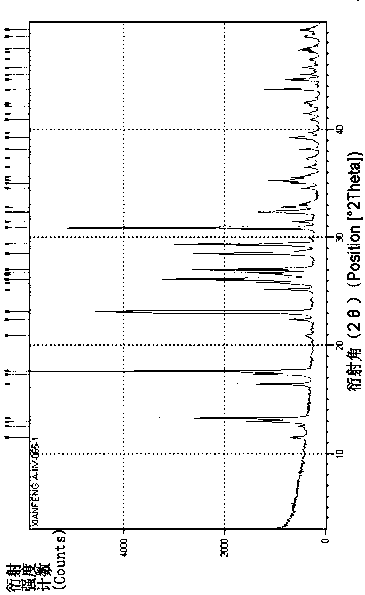
Phosphine and sodium trimetaphosphate preparation method
A technology of sodium trimetaphosphate and phosphine, applied in chemical instruments and methods, phosphine, phosphorus compounds, etc., can solve the problems of low phosphine yield, consumption of large element phosphorus, environmental pollution, etc. Reduce the production of long-chain high molecular weight chemicals, increase the content, and ensure the effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A mixture of sodium phosphite and sodium phosphate prepared from 25 g of phosphorous acid, 26 g of phosphoric acid (85.7%) and 21 g of sodium hydroxide (96%) was evaporated to dryness under reduced pressure at 70°C to constant weight to obtain the theoretical amount of trivalent phosphorus sodium salt and pentavalent phosphorus sodium salt anhydrous mixture 55.8g, the P of the mixed salt III / P V Calculated value is 1.34, and the sodium-phosphorus ratio calculated value is 0.947, and the pH value of the solution of 1% is 3.34 with the concentration that this mixed salt is made, and this mixed salt 30g is placed in the there-necked flask that nitrogen inlet and outlet are housed, with Replace the air in the flask with nitrogen. After half an hour, keep the nitrogen flow, the pressure is slightly higher than the standard atmospheric pressure, and then raise the temperature of the mixed salt to 400oC. In the absorption bottle of mercury solution (2-3%), the released phosp...
Embodiment 2
[0027] Sodium phosphite and sodium phosphate salt mixture (P III / P V=1.001) evaporated to dryness under reduced pressure at 70°C to constant weight to obtain 54.3 grams of theoretical anhydrous mixed salt. The calculated value of the sodium-phosphorus ratio of the mixed salt is 0.950. The pH value of solution is 3.48, and this mixed salt 10g is placed in the there-necked flask that nitrogen inlet and outlet are housed, replaces air in the flask with nitrogen, after half an hour, keeps nitrogen flow, and pressure is slightly greater than standard atmospheric pressure, then mixture is heated up to 350°C, the material melts and bubbles at this temperature, the released phosphine is brought into the absorption bottle filled with mercury chloride solution (2-3%) by nitrogen, and the released phosphine is dried by the precipitate in the absorption liquid The final weight and the amount of mercury ion loss in the solution are determined. After the bubbling ends, continue heating at...
Embodiment 3
[0029] Sodium phosphite and sodium phosphate salt mixture (P III / P V =1.000) evaporated to dryness under reduced pressure at 70°C to constant weight to obtain 90.2 grams of theoretical anhydrous mixed salt. The calculated value of the sodium-phosphorus ratio of the mixed salt is 0.990. The pH value of solution is 3.51, and this mixed salt 20g is placed in the there-necked flask that nitrogen inlet and outlet are housed, replaces air in the flask with nitrogen, after half an hour, keeps nitrogen flow, and pressure is slightly greater than standard atmospheric pressure, then mixture is heated up to 310°C, the material melts and bubbles at this temperature, and the released phosphine is brought into the absorption bottle filled with mercuric chloride solution (2-3%) by nitrogen gas, and the released phosphine is dried by the precipitate in the absorption liquid The final weight and the amount of mercury ion loss in the solution are determined. After the bubbling ends, continue ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com