Tetracarboxylic acid perylene diimide compound, preparation method and application thereof
A compound, tetrachloroperylene anhydride technology, applied in tetracarboxylic acid perylene diimide compound and its preparation and application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1. Preparation of N, N'-bis(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrabromo-3,4:9,10-tetracarboxylic acid Dianhydride
[0109]
[0110] To 10mmol of tetrachloroperylene anhydride and 30mmol of DBI, add 300ml of fuming sulfuric acid with a mass percentage of sulfur trioxide of 50%, and react at 50°C. After 1d, the mixture is cooled to room temperature and slowly poured into ice. The Buchner funnel was suction-filtered, washed with a large amount of water and ethanol, and dried in a vacuum oven to obtain a crude product, which was directly used in the next reaction with a yield of 90%. Red powder, MS (MALDI-TOF, m / z): C25H2Br4Cl4O5, 845.5, Anal. Calcd for: 845.5 (100.0%). The mass spectrum of N, N'-di(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrabromo-3,4:9,10-tetracarboxylic dianhydride is as follows figure 1 shown. It can be seen from the figure that the product has a correct structure and is the target compound.
Embodiment 2
[0111] Example 2. Preparation of N, N'-bis(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrabromo-3,4:9,10-tetracarboxylic acid Diimide
[0112]
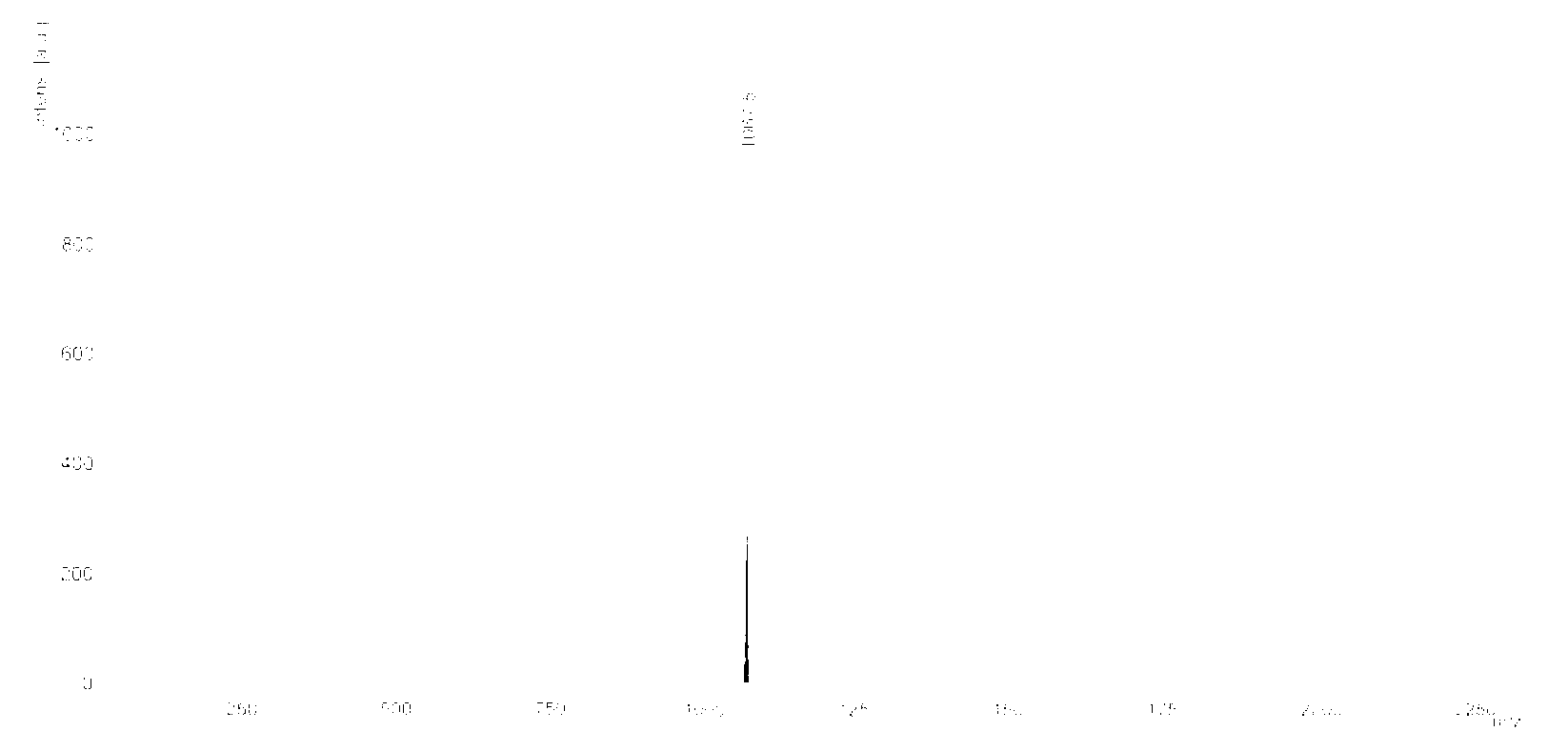
[0113] Add 5 mmol of the product prepared in Example 1 above and 5 ml of n-octylamine to 300 ml of acetic acid, reflux for 24 hours under an inert gas atmosphere, cool to room temperature, pour into 300 ml of water, filter with Buchner funnel, and wash with water. Drying gave the crude product. Separation and purification through silica gel to obtain N,N'-di(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrabromo-3,4:9,10-tetra Carboxylic acid diimide. Yield is 50%, red powder, C40H34Br4Cl4N2O4, 1067.8, Anal.Calcd for: 1067.5 (100.0%)
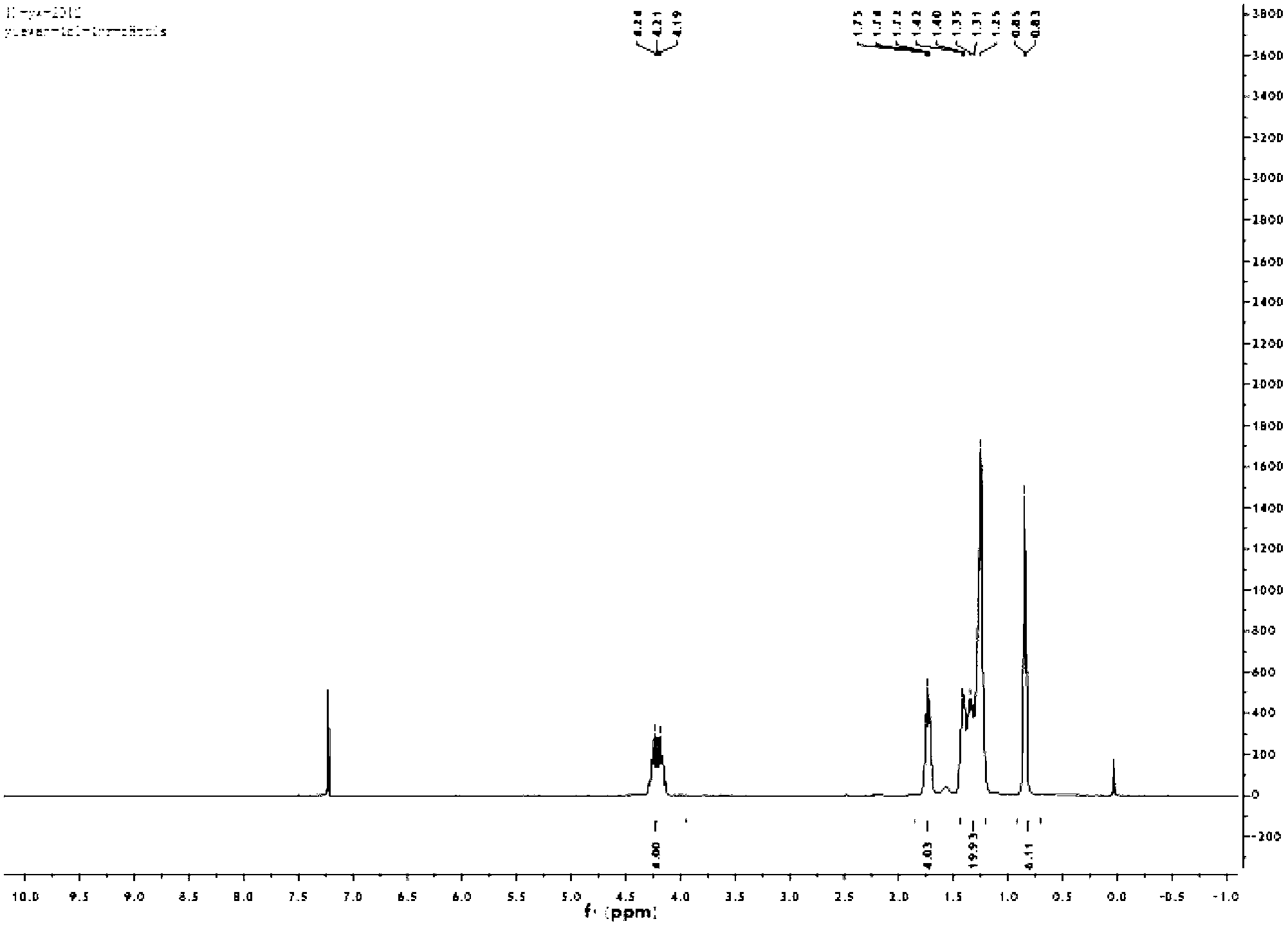
[0114] N,N'-di(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrabromo-3,4:9,10-tetracarboxylic diimide The mass spectrum is shown in Figure 2; 1 H-NMR picture as image 3 Shown; UV-Vis absorption diagram as Figure 4 Shown; the electrochemical diagram is shown in Figure 5 shown. The formation of the tar...
Embodiment 3
[0115] Example 3. Preparation of N, N'-bis(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrafluoro-3,4:9,10-tetracarboxylic acid Diimide
[0116]
[0117] To 1mmol N,N'-di(n-octyl)-2,5,8,11-tetrabromo-1,6,7,12-tetrachloro 3,4:9,10-tetracarboxylic perylene diimide Amine compound, 20mmol potassium fluoride, 0.5mmol phase transfer catalyst 18-crown-6 were reacted in 1ml sulfolane solvent at 150°C for 2h, the mixture was cooled to room temperature, poured into water, suction filtered to obtain filter cake, and dried. Separation and purification by silica gel column chromatography to obtain the orange compound N,N'-di(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrafluoro-3,4:9,10 - Tetracarboxylic perylene diimide compound, yield 30%, MS (MALDI-TOF, m / z): C40H34Cl4F4N2O4, 824.1, Anal. Calcd for: 824.1.
[0118] N,N'-bis(n-octyl)-1,6,7,12-tetrachloro-2,5,8,11-tetrafluoro-3,4:9,10-tetracarboxylic diimide Mass spectrometry such as Figure 6 shown; 1 H-NMR picture as Figure 7 Shown; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap