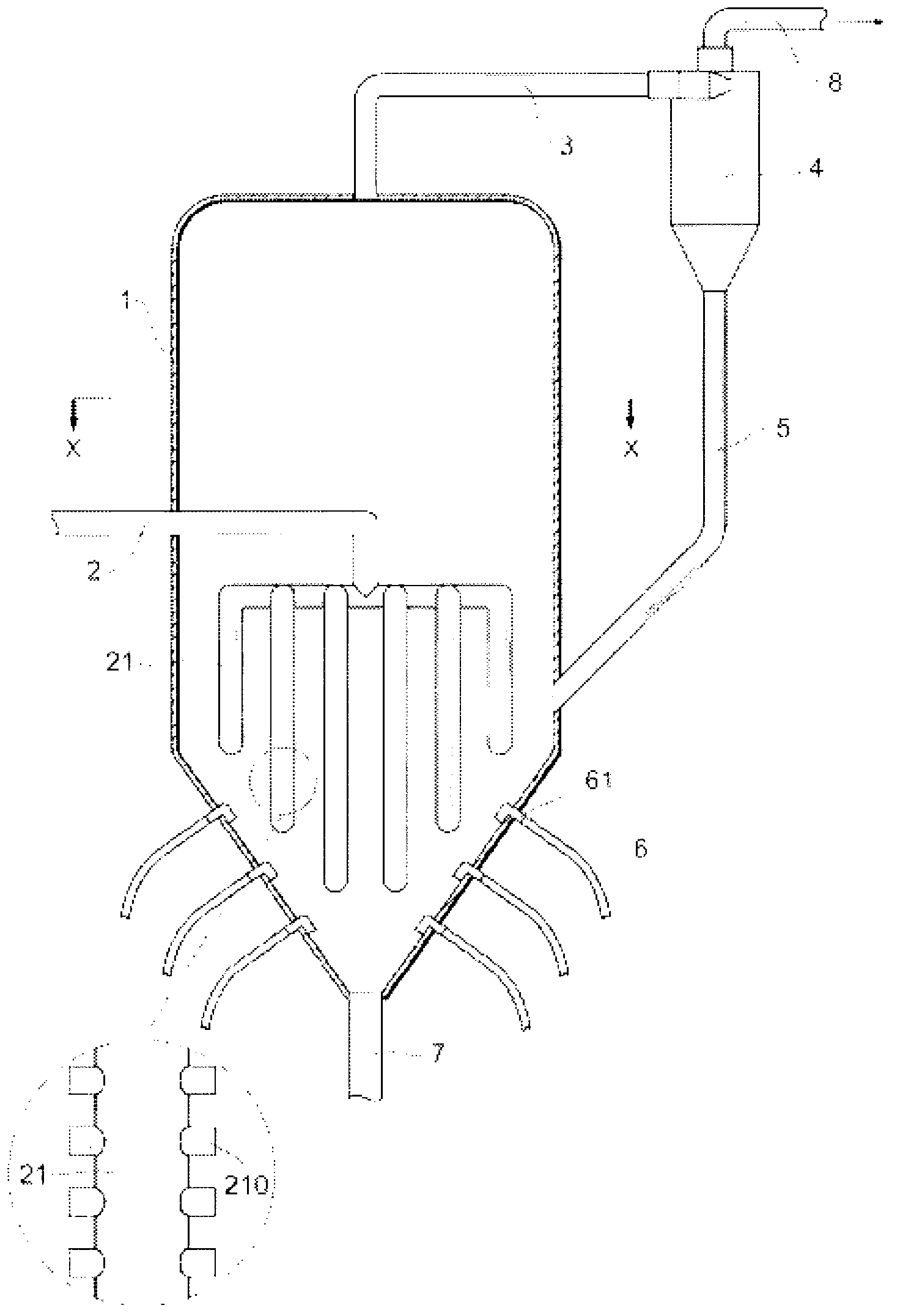
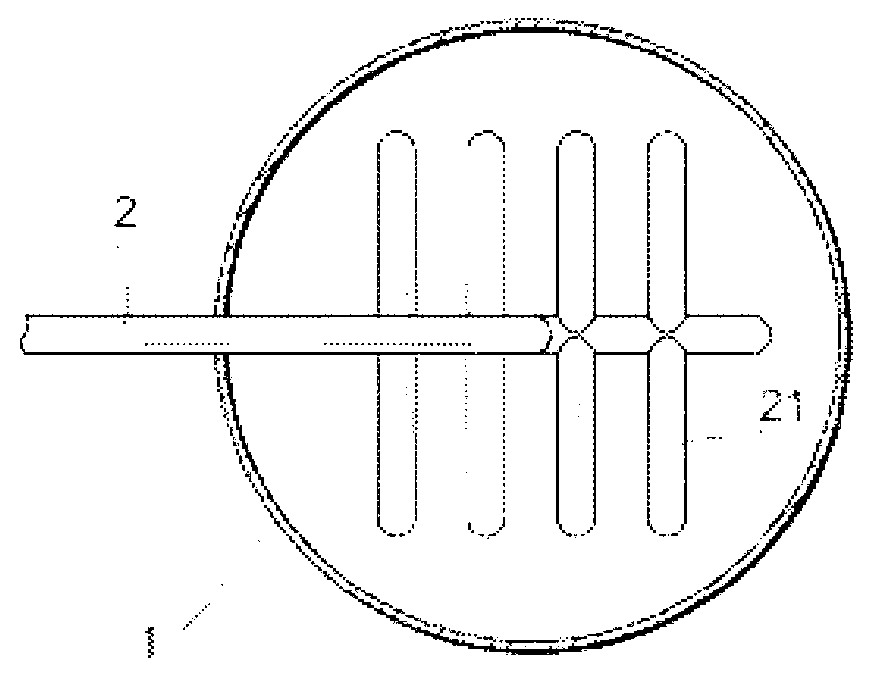
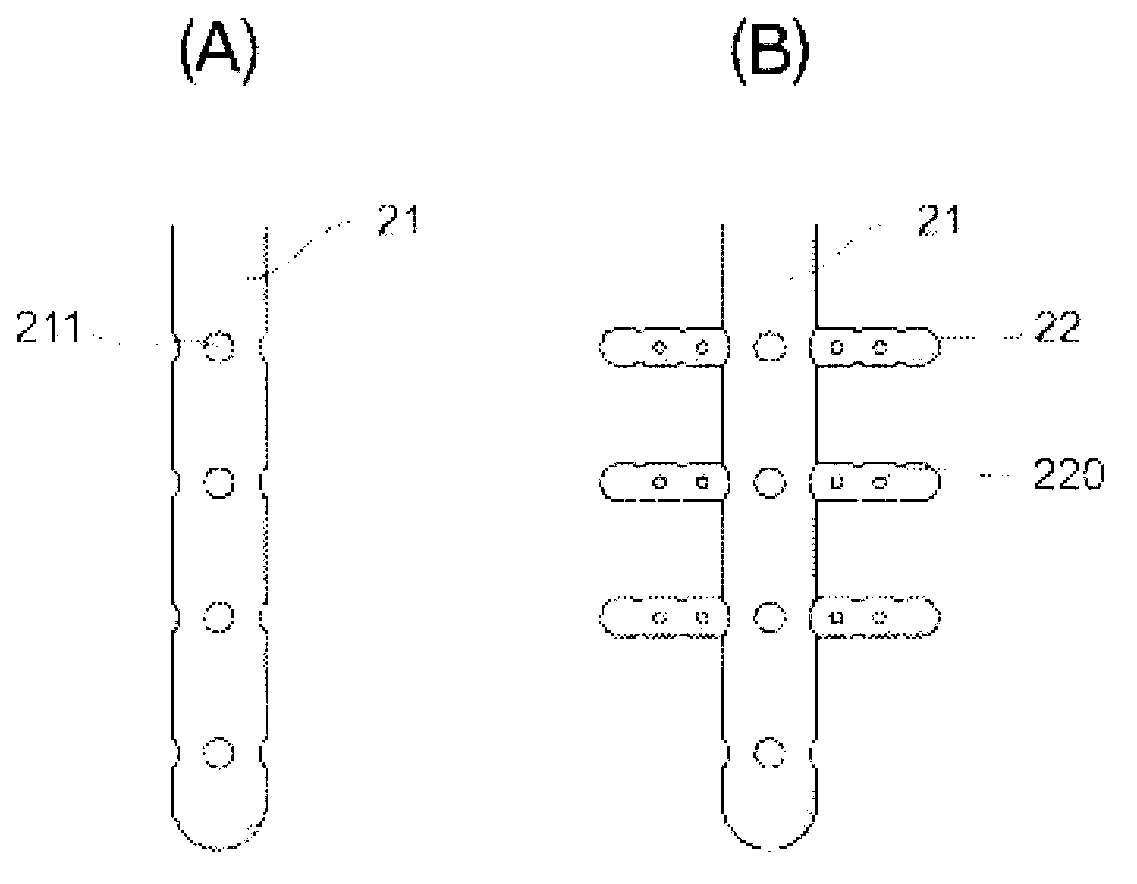
Composite oxide catalyst and method for producing same
A composite oxide and manufacturing method technology, applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve the problems of metal oxides affecting catalyst performance, etc. The effect of good catalyst performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0325] (manufacture of dry powder)
[0326] dry powder (D 1 ) is manufactured as follows.
[0327] Add 432.1g ammonium heptamolybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O], 59.9g ammonium metavanadate [NH 4 VO 3 ], 84.3g antimony trioxide [Sb 2 o 3 ] and 4.8g cesium nitrate [Ce(NO 3 ) 3 ·6H 2 O], heated at 95° C. for 1 hour while stirring, thereby producing an aqueous raw material solution (A 1 ).
[0328] To 378.4g niobium mixture (B 0 ) containing 30% by mass H 2 o 2 66.3g of hydrogen peroxide solution, stirred and mixed at room temperature for 10 minutes, thereby making aqueous raw material solution (B 1 ).
[0329] The obtained aqueous raw material solution (A 1 ) after cooling to 70°C, to the aqueous raw material solution (A 1 ) containing 34.0 mass% SiO 2 807.8g of silica sol, further adding 30% by mass H 2 o 2 98.4 g of hydrogen peroxide solution was stirred continuously for 30 minutes at 55°C. Next, to this liquid, sequentially add the aqueous raw materi...
Embodiment 2
[0342] (manufacture of dry powder)
[0343] dry powder (D 1 ) is manufactured as follows.
[0344] Add 611.5g ammonium heptamolybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O], 84.7g ammonium metavanadate [NH 4 VO 3 ], 119.3g antimony trioxide [Sb 2 o 3 ] and 6.8g cesium nitrate [Ce(NO 3 ) 3 ·6H 2 O], heated at 95° C. for 1 hour while stirring, thereby producing an aqueous raw material solution (A 1 ).
[0345] To 535.5g niobium mixture (B 0 ) containing 30% by mass H 2 o 2 93.8g of hydrogen peroxide solution, stirred and mixed at room temperature for 10 minutes, thereby making aqueous raw material solution (B 1 ).
[0346] The obtained aqueous raw material solution (A 1 ) after cooling to 70°C, to the aqueous raw material solution (A 1 ) containing 34.0 mass% SiO 2 429.7g of silica sol, further adding 30% by mass H 2 o 2 98.4 g of hydrogen peroxide solution was stirred continuously for 30 minutes at 55°C. Next, to this liquid, sequentially add the aqueous raw mater...
Embodiment 3
[0357] (manufacture of dry powder)
[0358] dry powder (D 1 ) is manufactured as follows.
[0359] Add 285.4g ammonium heptamolybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O], 39.5g ammonium metavanadate [NH 4 VO 3 ], 55.7g antimony trioxide [Sb 2 o 3 ] and 3.2g cesium nitrate [Ce(NO 3 ) 3 ·6H 2 O], heated at 95° C. for 1 hour while stirring, thereby producing an aqueous raw material solution (A 1 ).
[0360] To 249.9g niobium mixture (B 0 ) containing 30% by mass H 2 o 2 43.8g of hydrogen peroxide solution, stirred and mixed at room temperature for 10 minutes, thereby making aqueous raw material solution (B 1 ).
[0361] The obtained aqueous raw material solution (A 1 ) after cooling to 70°C, to the aqueous raw material solution (A 1 ) containing 34.0 mass% SiO 2 1117.2g of silica sol, further adding 30% by mass H 2 o 2 98.4 g of hydrogen peroxide solution was stirred continuously for 30 minutes at 55°C. Next, to this liquid, sequentially add the aqueous raw mater...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Crystal size | aaaaa | aaaaa |
| Crystal size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com