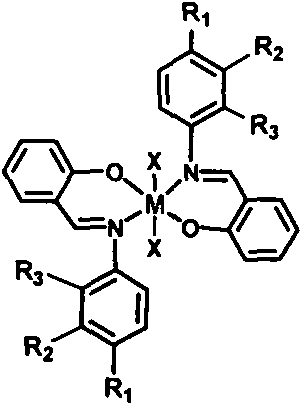
Schiff base metal catalyst used in liquid phase epoxidation reaction and preparation method of schiff base metal catalyst
A metal catalyst, epoxidation reaction technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc. Alkali ligands are expensive and the active components are easy to dissolve, which can achieve the effects of stable reuse performance, convenient recovery and high activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 150mL of acetonitrile, 6.85g of p-aminobenzoic acid and 6.10g of salicylaldehyde into a 200mL round bottom flask, and stir for 1h at room temperature. The solid was filtered out, and after recrystallization with ethanol, the obtained solid product was dried at 80° C. for 12 hours to obtain 9.8 g of Schiff base ligand (A). Add 4.82 g of the obtained Schiff base ligand and 2.66 g of oxymolybdenum acetylacetonate into 40 mL of acetonitrile solvent, stir at 80°C for 24 hours, filter, wash, and dry at 80°C to obtain the A-molybdenum catalyst. NMR characteristic peaks (300Hz, CDCl 3 )d=7.02(m, 2H), 7.45(m, 3H), 7.70(d, 1H), 8.05(d, 2H), 9.00(s, 1H), 10.45(s, 1H), 12.85(s, 1H ).
[0016] In a 50mL round-bottomed flask equipped with a magnetic stirrer and a spherical condenser, add 0.06g of catalyst A-molybdenum, 15mL of chloroform solvent, 5mmol of cyclooctene and 15mmol of tert-butyl hydroperoxide (TBHP), and heat the water bath to 70°C. Reaction 4h. Then GC analysis ...
Embodiment 2
[0018] Under the situation that the preparation conditions of the catalyst are exactly the same as those in Example 1, only the oxymolybdenum acetylacetonate in the preparation method is replaced with vanadyl acetylacetonate to obtain the catalyst A-vanadium. Catalyst A-vanadium was used for the epoxidation of cyclooctene according to the method described in Example 1, and the olefin conversion rate and product selectivity were both 100%.
Embodiment 3
[0020] Under the situation that the preparation conditions of the catalyst are exactly the same as in Example 1, only the p-aminobenzoic acid in the preparation method is replaced with aniline to obtain the catalyst B-molybdenum. Catalyst B-molybdenum was used in the epoxidation reaction of cyclooctene according to the method described in Example 1, and the olefin conversion rate and product selectivity were both 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com