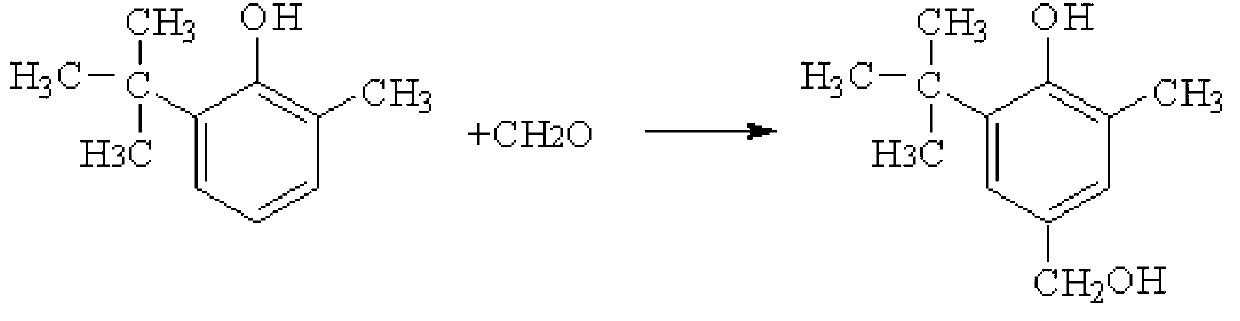
Preparation method of hindered phenol antioxidant 2-methyl-4-hydroxymethyl-6-tert-butyl phenol
A technology of tert-butylphenol and methylol, applied in the field of polymer material preparation, can solve the problems of reduced dosage, high volatility, low molecular weight, etc., and achieve the effects of low volatility, mild reaction conditions and good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment provides a kind of 2-methyl-4-hydroxymethyl-6-tert-butylphenol, which is prepared through the following steps:
[0028] Add 80mL of tert-butanol, 2.4g of paraformaldehyde, 6.56g of 2-methyl-6-tert-butylphenol and 0.3g of catalyst potassium hydroxide into a 250mL three-necked flask equipped with a stirrer, a nitrogen conduit and a thermometer, get the mixture;
[0029] Purging with nitrogen, stirring, heating to 82°C for 2.5 hours at a constant temperature, after the reaction, add 80mL of distilled water into the three-necked flask for vacuum distillation;
[0030] Then separate out the solid to obtain the crude product;
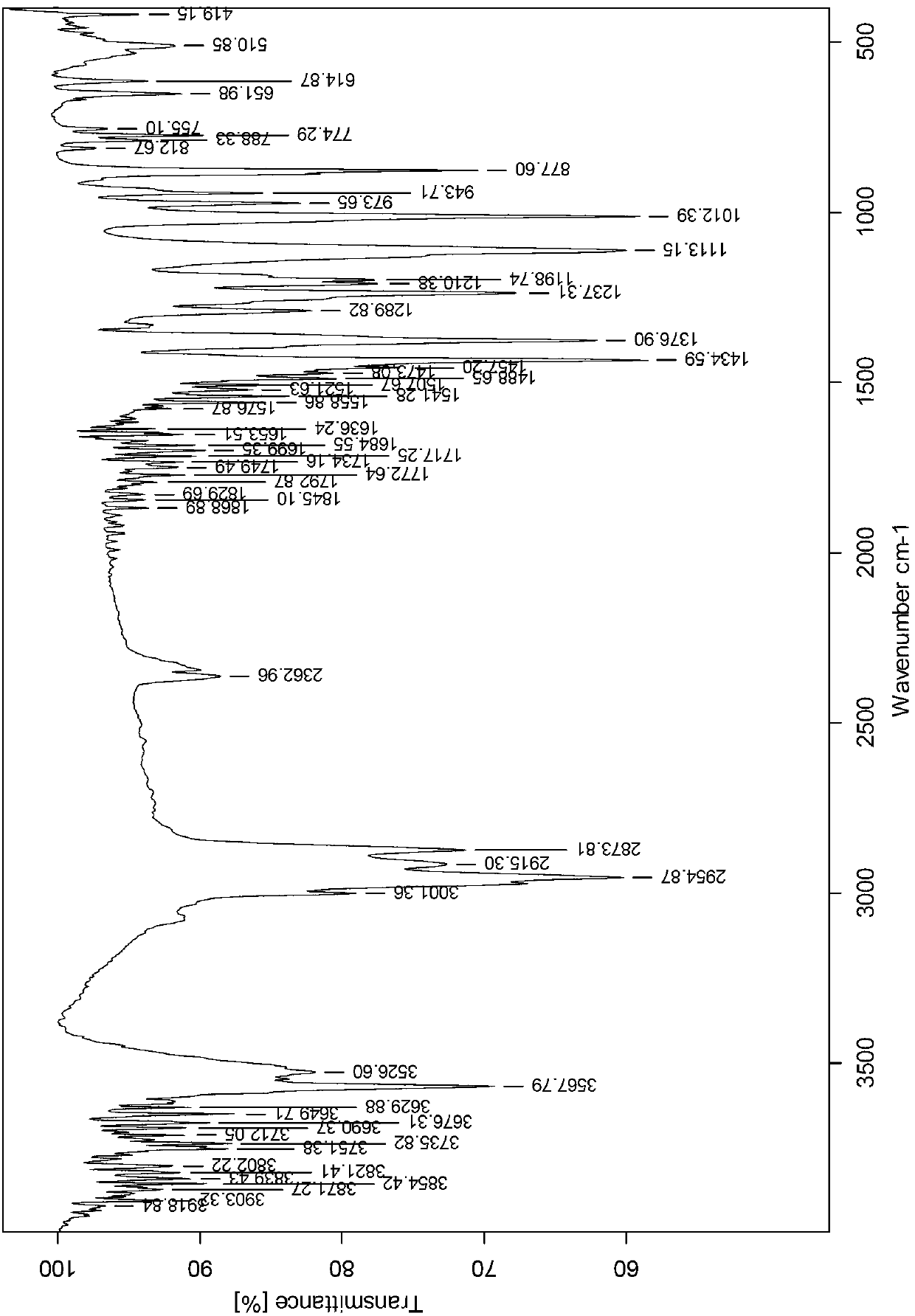
[0031] The crude product was recrystallized twice with 60mL isopentane to obtain pure 2-methyl-4-hydroxymethyl-6-tert-butylphenol with a melting point of 125.2°C. For the reaction principle, see figure 1 , see the infrared spectrum figure 2 .
Embodiment 2
[0033] This embodiment provides a kind of 2-methyl-4-hydroxymethyl-6-tert-butylphenol, which is prepared through the following steps:
[0034] 270mL of tert-butanol, 6.2g of paraformaldehyde, 16.4g of 2-methyl-6-tert-butylphenol and 0.45g of catalyst potassium hydroxide were sequentially added to a 1000mL three-necked flask equipped with a stirrer, a nitrogen conduit and a thermometer, get the mixture;
[0035] Purging with nitrogen, stirring, heating to 82°C for 3 hours at a constant temperature, after the reaction, add 150mL of distilled water into the three-necked flask for vacuum distillation;
[0036] Then separate out the solid to obtain the crude product;
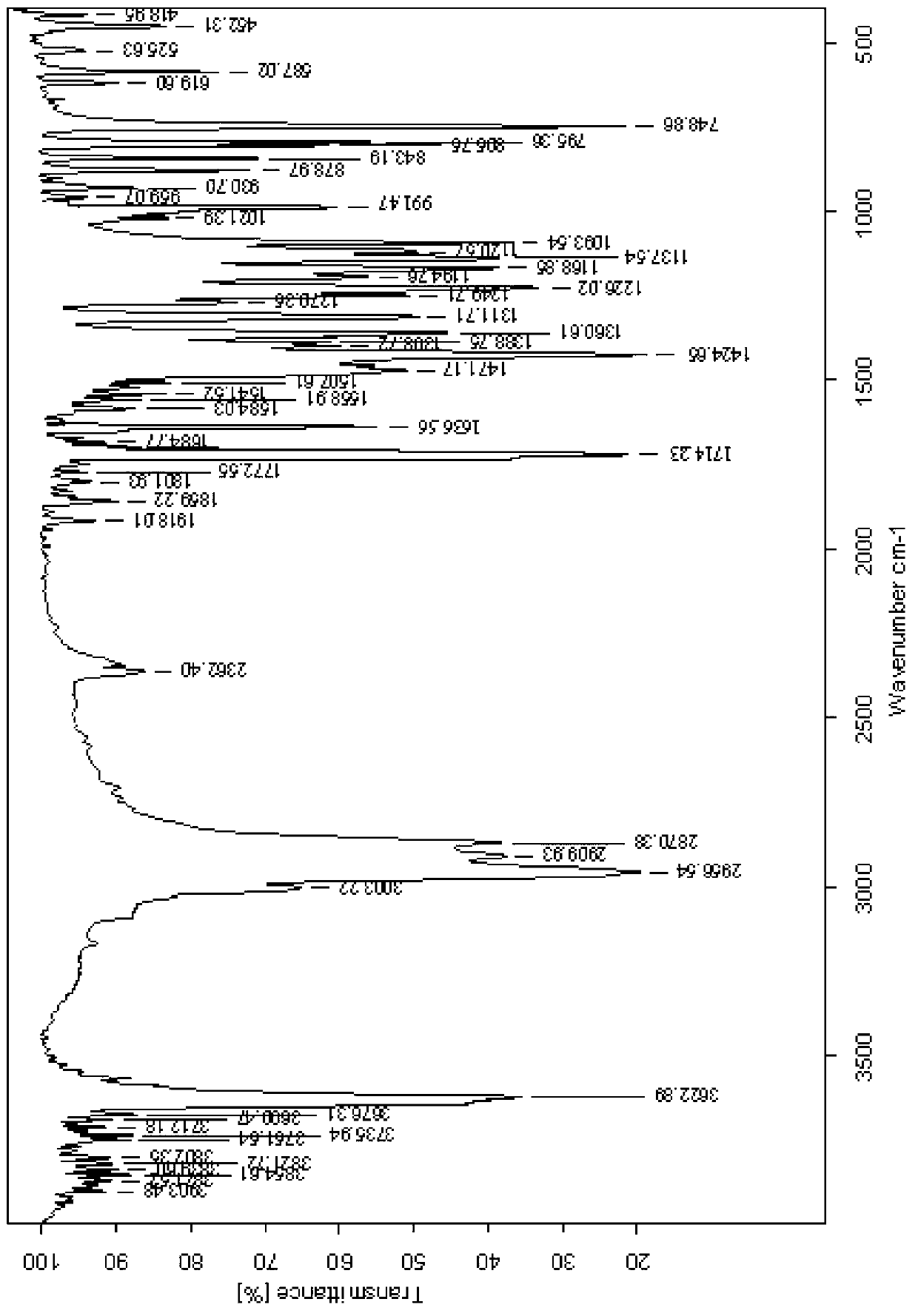
[0037] The crude product was recrystallized twice with 260mL isopentane to obtain pure 2-methyl-4-hydroxymethyl-6-tert-butylphenol with a melting point of 125.2°C. For the reaction principle, see figure 1 , see the infrared spectrum image 3 .
Embodiment 3
[0039] This embodiment provides a kind of 2-methyl-4-hydroxymethyl-6-tert-butylphenol, which is prepared through the following steps:
[0040] 590mL of tert-butanol, 13.5g of paraformaldehyde, 32.8g of 2-methyl-6-tert-butylphenol and 1.0g of catalyst potassium hydroxide were sequentially added to a 1000mL three-necked flask equipped with a stirrer, a nitrogen conduit and a thermometer, get the mixture;
[0041] Purging with nitrogen, stirring, heating to 82°C for 3.5 hours at a constant temperature, after the reaction, add 300mL of distilled water into the three-necked flask for vacuum distillation;
[0042] Then separate out the solid to obtain the crude product;
[0043] The crude product was recrystallized twice with 500mL isopentane to obtain pure 2-methyl-4-hydroxymethyl-6-tert-butylphenol with a melting point of 124.6°C and an infrared spectrum shown in Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com