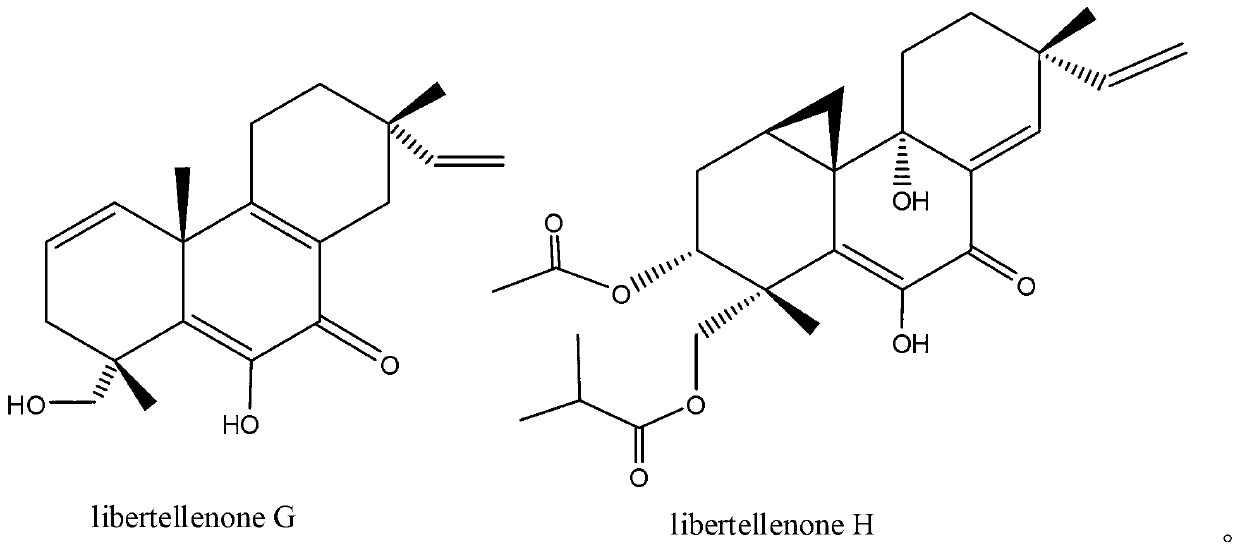
Diterpenoid compounds libertellenone G and libertellenone H with antineoplastic activities and application thereof
A kind of technology of compound and diterpenes, applied in the field of application in the preparation of antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Fermentation culture of Curvularia sp. D-1 (CCTCC M2013144)
[0027] a. Curvularia sp.D-1 (Eutypella sp.D-1), was preserved in the China Center for Type Culture Collection (CCTCC) on April 12, 2013, with the preservation number CCTCC M2013144; Curvularia sp.D-1 The fermentation medium of fungus D-1 is as follows: 10 g of potato extract powder, 20 g of glucose, and 1000 mL of distilled water.
[0028] b. Fermentation culture process: D-1 picks a small amount of mycelia of the D-1 strain from the plate, inoculates it into a 250mL Erlenmeyer flask containing PDB medium for seed cultivation, and each Erlenmeyer flask contains 70mL of medium, 20 Cultivate at constant temperature on a shaking table at 180r / min. After 5 days of cultivation, inoculate the seed culture solution into a 2000mL Erlenmeyer flask for expanded cultivation. Fill each bottle with 500mL of PDB medium, inoculate at 12% inoculum, and cultivate for 9 days under the same cultivation conditions.
Embodiment 2
[0029] Embodiment 2: Separation and extraction of active compound
[0030] The bacterial liquid that has completed the fermentation and cultivation process is filtered, and the supernatant of the fermentation liquid is collected after removing the mycelium. The supernatant of the fermentation broth was extracted with an equal volume of ethyl acetate, repeated three times, the combined extracts were evaporated to dryness with a rotary evaporator, the extract was roughly segmented with Sephadex LH-20, and the mobile phase was methanol. The obtained eluted phase was evaporated to dryness and then purified by ODS reverse-phase column chromatography. The mobile phase was: methanol-water. The eluted part of 90% methanol-water was collected, concentrated and then continued to pass ODS reverse-phase column chromatography. The mobile phase was : Methanol-water, the elution fraction of 70% methanol-water was collected, concentrated and used preparative TLC plate (HSGF254, Yantai Zhifu H...
Embodiment 3
[0040] Example 3: Inhibitory Effects of Active Compounds Libertellenone G and Libertellenone H on Human Cancer Cell Lines
[0041] Method: MTT method (Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods, 1983, 65, 55-63.):
[0042] Human glioma cell line U251 in logarithmic growth phase, human pancreatic cancer cell line SW1990, human gastric cancer cell line SG7901, human breast cancer cell line Mcf-7, human liver cancer cell line Huh-7, and human cervical cancer cell line Hela, human lung cancer cell line H460, each with 2×10 4 Cells per well were seeded on a 96-well plate, and different concentrations (1.5 μg / ml—50 μg / ml) of libertellenone G and libertellenone H were added, and the 0.1% DMSO control group was cultured for 72 hours, and 5 mg / mL MTT10 μL was added to each well 4 hours before the end. At the end of the culture, centrifuge and carefully suck off the supernatant, add 200 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com