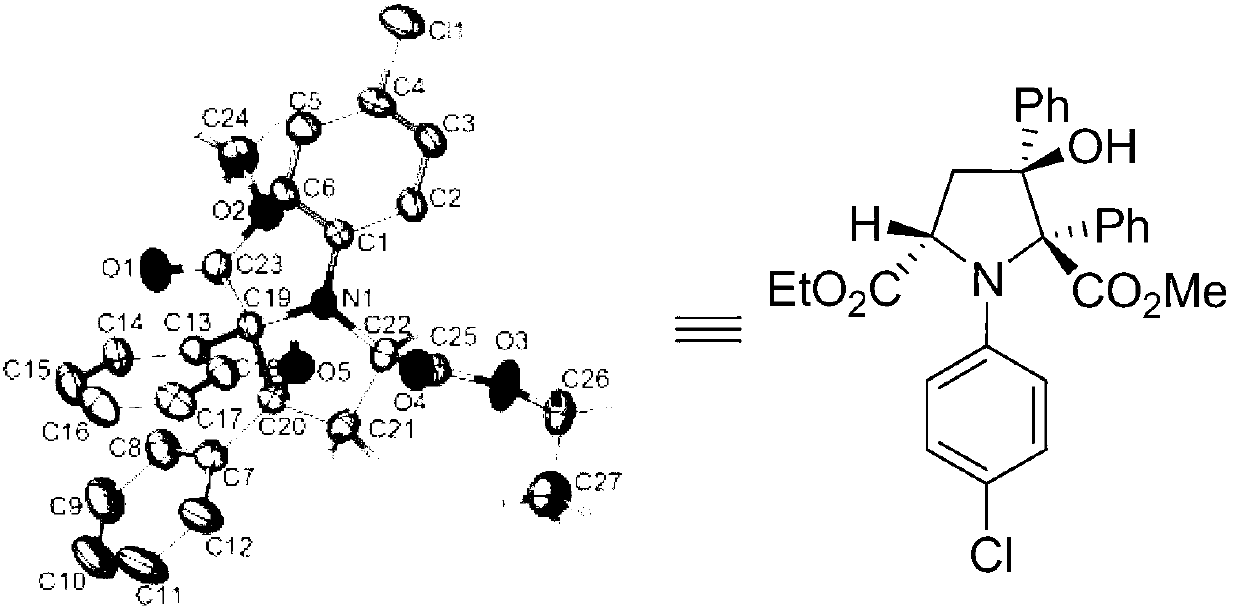
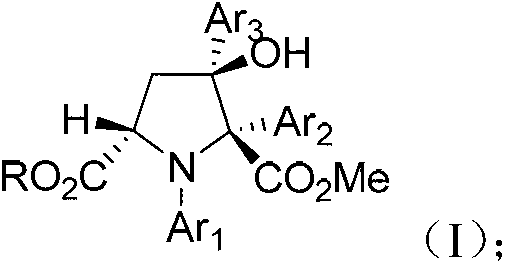
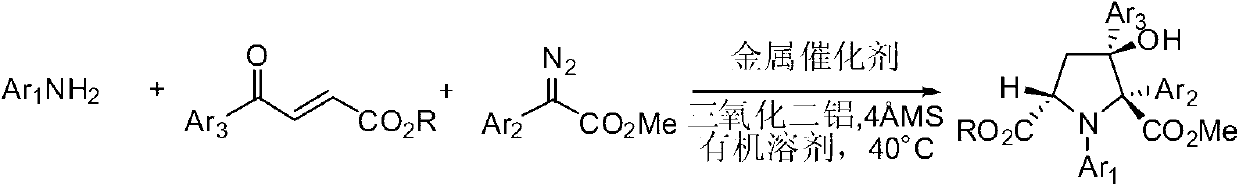
Synthetic method for 3-hydroxyl multi-substituted tetrahydropyrrole derivative
A technology of tetrahydropyrrole and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of low synthesis yield and many synthesis steps, and achieve the effects of mild reaction conditions, good selectivity and wide applicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Aniline (0.30mmol), methyl 4-carbonyl-4-phenyl-2-butenoate (0.36mmol), basic aluminum oxide (0.30mmol), A mixture of molecular sieves (300 mg) and rhodium acetate (0.3 mmol%) was dissolved in 1.5 mL of dichloromethane solvent to prepare a mixed solution A, which was stirred at 40° C. for 1 hour. Then, methyl 2-diazo-2-phenylacetate (0.54 mmol) was dissolved in 1.5 mL of dichloromethane solvent to prepare solution B. Solution B was added to mixed solution A with a syringe pump at 40°C within 1 hour. The reaction mixture was purified by flash column chromatography to obtain pure product. The yield was 88%, and the dr value was greater than 93:7.
[0035] 1 H NMR (400MHz, CDCl 3 )δ7.48(br.s, 2H), 7.23(d, J=7.2Hz, 1H), 7.17-7.11(m, 5H), 7.09-7.03(m2H), 6.97(d, J=7.4Hz, 1H ), 6.75(t, J=6.9Hz, 1H), 6.44(d, J=7.7Hz, 2H), 5.08(dd, J=10.4, 4.8Hz, 1H), 4.54(s, 1H), 3.84(s , 3H), 3.58(s, 3H), 2.79(dd, J=11.5, 11.3Hz, 1H), 2.36(dd, J=11.6, 4.8Hz, 1H); 13 C NMR (1...
Embodiment 2
[0037]
[0038] Aniline (0.30mmol), methyl 4-carbonyl-4-phenyl-2-butenoate (0.36mmol), basic aluminum oxide (0.30mmol), A mixture of molecular sieves (300 mg) and rhodium propionate (0.3 mmol%) was dissolved in 1.5 mL of dichloromethane solvent to prepare a mixed solution A, which was stirred at 40° C. for 1 hour. Then, methyl 2-diazo-2-phenylacetate (0.54 mmol) was dissolved in 1.5 mL of dichloromethane solvent to prepare solution B. Solution B was added to mixed solution A with a syringe pump at 40°C within 1 hour. The reaction mixture was purified by flash column chromatography to obtain pure product. The yield was 90%, and the dr value was greater than 90:10.
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.48(br.s, 2H), 7.23(d, J=7.2Hz, 1H), 7.17-7.11(m, 5H), 7.09-7.03(m2H), 6.97(d, J=7.4Hz, 1H ), 6.75(t, J=6.9Hz, 1H), 6.44(d, J=7.7Hz, 2H), 5.08(dd, J=10.4, 4.8Hz, 1H), 4.54(s, 1H), 3.84(s , 3H), 3.58(s, 3H), 2.79(dd, J=11.5, 11.3Hz, 1H), 2.36(dd, J=11.6, 4.8Hz, 1H); 13 C N...
Embodiment 3
[0041]
[0042] Aniline (0.30mmol), methyl 4-carbonyl-4-phenyl-2-butenoate (0.36mmol), acidic aluminum oxide (0.30mmol), A mixture of molecular sieves (300 mg) and rhodium acetate (0.3 mmol%) was dissolved in 1.5 mL of chloroform solvent to prepare a mixed solution A, which was stirred at 40° C. for 1 hour. Then, methyl 2-diazo-2-phenylacetate (0.54 mmol) was dissolved in 1.5 mL of chloroform solvent to prepare solution B. Solution B was added to mixed solution A with a syringe pump at 40°C within 1 hour. The reaction mixture was purified by flash column chromatography to obtain pure product. The yield was 80%, and the dr value was greater than 91:9.
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.48(br.s, 2H), 7.23(d, J=7.2Hz, 1H), 7.17-7.11(m, 5H), 7.09-7.03(m2H), 6.97(d, J=7.4Hz, 1H ), 6.75(t, J=6.9Hz, 1H), 6.44(d, J=7.7Hz, 2H), 5.08(dd, J=10.4, 4.8Hz, 1H), 4.54(s, 1H), 3.84(s , 3H), 3.58(s, 3H), 2.79(dd, J=11.5, 11.3Hz, 1H), 2.36(dd, J=11.6, 4.8Hz, 1H); 13 C NMR (100MHz, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com