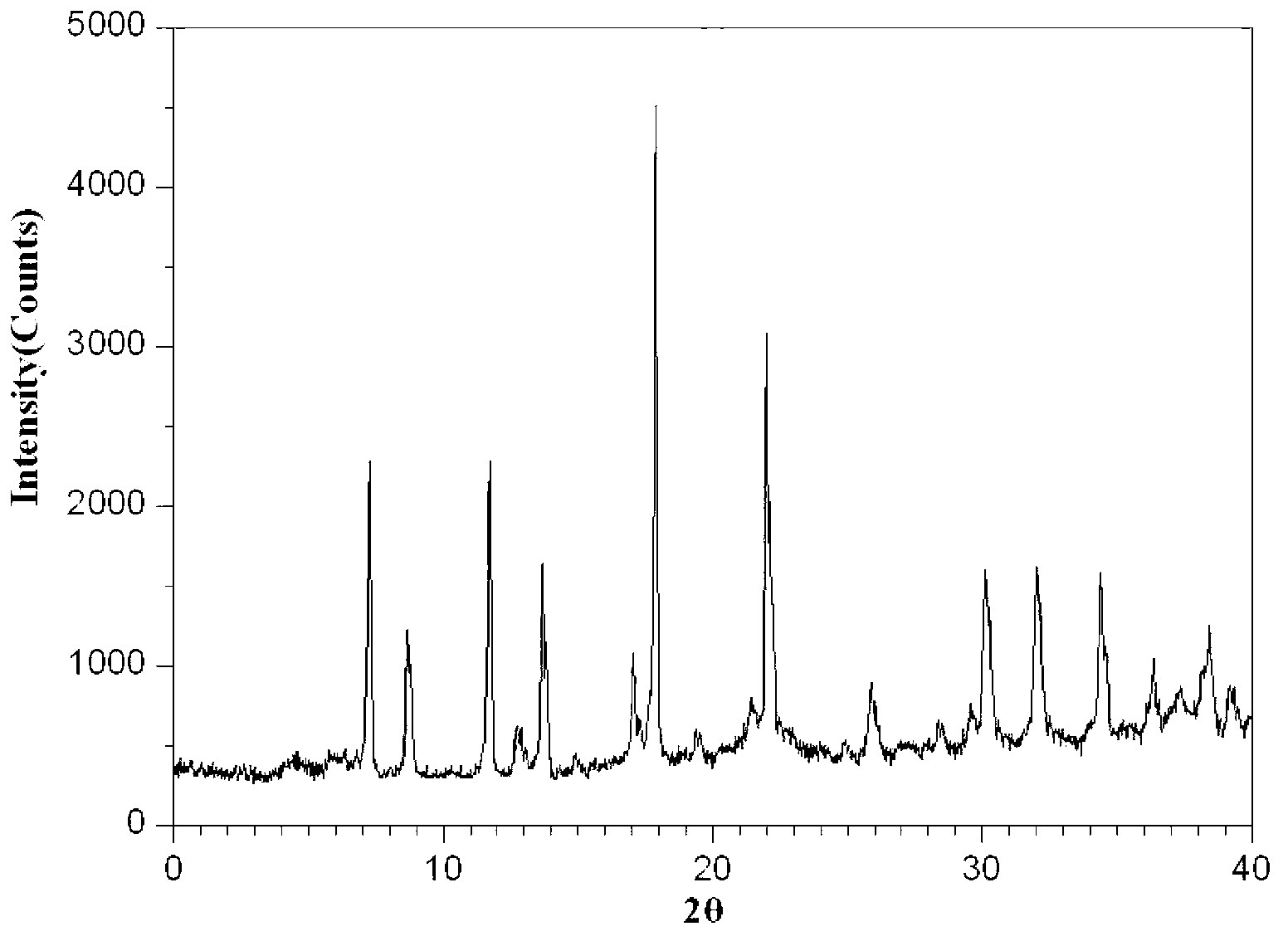
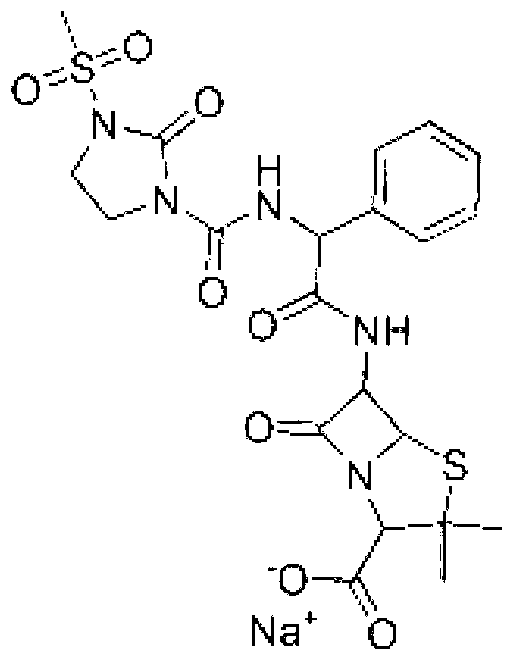
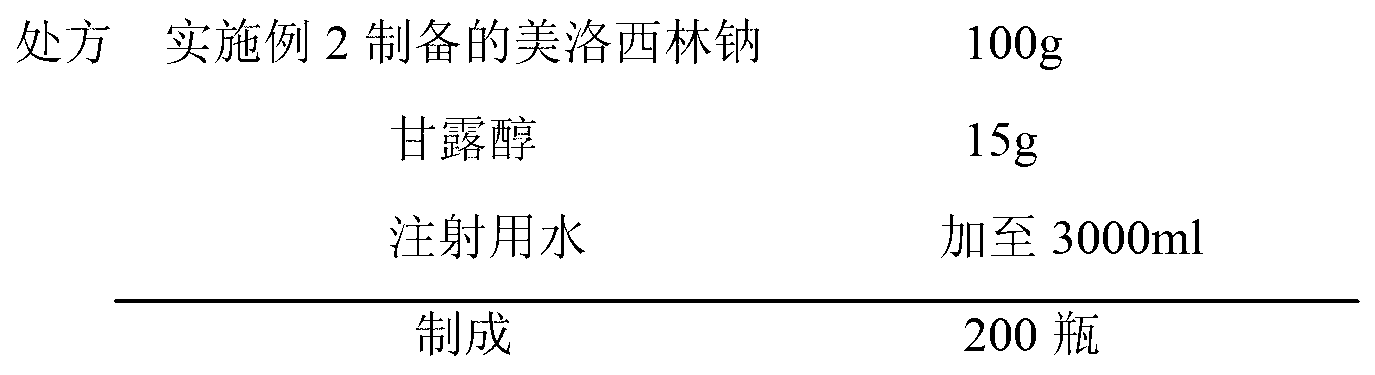
Mezlocillin sodium compound as well as preparation method and pharmaceutical composition thereof
A technology of mezlocillin sodium and compound, applied in the field of medicine, can solve the problems of slow dissolution rate and poor fluidity, and achieve the effects of fast dissolution rate, good fluidity and convenient compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation method of mezlocillin sodium compound:
[0047] N,N-dimethylformamide and methanol are formulated into a mixed solvent at a volume ratio of 2:1, the raw material drug of mezlocillin sodium is taken, and the mixed solvent of N,N-dimethylformamide and methanol is added, and the The ratio of the volume of the mixed solvent to the mass of mezlocillin sodium is 5ml: 1g, stir, adjust the pH of the solution to 6.5, add activated carbon for decolorization, the amount of added activated carbon is 0.2% g / ml of the total volume of the medicinal solution, stir After 30 minutes, filter to obtain a clear solution. Move the clear solution into a reaction kettle, place it in a 100°C oven for 48h, cool down to 70°C at a rate of 5°C / h, open the kettle, stir slowly and add ether dropwise. The stirring rate was 15 rpm, the amount of diethyl ether was 5 times that of the mixed solvent, the temperature was lowered to 0°C, filtered, washed 3 times with diethyl ether, and then ...
Embodiment 2
[0050] The preparation method of mezlocillin sodium compound:
[0051] N,N-dimethylformamide and methanol are formulated into a mixed solvent at a volume ratio of 3:1, and the raw material drug of mezlocillin sodium is taken, and the mixed solvent of N,N-dimethylformamide and methanol is added, and the The ratio of the volume of the mixed solvent to the mass of mezlocillin sodium is 8ml: 1g, stir, adjust the pH of the solution to 5.5, add activated carbon for decolorization, the amount of added activated carbon is 0.3% g / ml of the total volume of the medicinal solution, stir After 25 minutes, filter to obtain a clear solution. Move the clear solution into a reaction kettle, place it in a 120°C oven for 12h, cool down to 60°C at a rate of 10°C / h, open the kettle, stir slowly and add ether dropwise. The stirring rate was 8rpm, the amount of diethyl ether was 3 times that of the mixed solvent, the temperature was lowered to 5°C, filtered, washed 3 times with diethyl ether, and th...
Embodiment 3
[0054] Preparation of mezlocillin sodium sterile powder injection:
[0055] Weigh 99.5 g of mezlocillin sodium and 0.5 g of sodium benzoate prepared in Example 1 under aseptic conditions, place them in a solid powder mixer and mix them evenly, and the raw materials obtained are transferred to the aseptic preparation workshop for precise metering and subpackaging, each bottle Containing 1.5g of mezlocillin sodium, stoppered and capped, the finished product is packaged for storage and sent for inspection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com