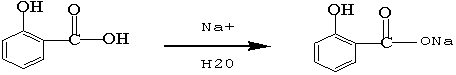
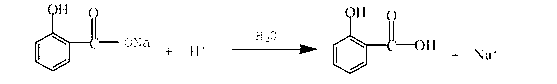Recycling method of 2-hydroxybenzoic acid-2-ethyl caprolactone production liquid waste
A technology of hydroxybenzoic acid and ethylhexyl ester, applied in the preparation of carboxylate, chemical instruments and methods, preparation of carboxylate, etc., can solve problems such as environmental pollution, increase the difficulty of wastewater treatment, waste resources, etc. Difficulty, high cost, and the effect of reducing processing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The mass concentration of the wastewater to be treated is 30% hydrochloric acid to adjust the pH value to 2, fully stir for half an hour, until all the salicylic acid is precipitated, the amount of hydrochloric acid is 50Kg, and a total of 70Kg of salicylic acid crystals are recovered, with a water content of 16%. The purity of salicylic acid measured by gas chromatography reached 98.6%; the recovered salicylic acid crystals were calculated and directly added to the reaction tank as raw materials to carry out the esterification synthesis of the next batch of 2-hydroxybenzoic acid 2-ethylhexyl.
Embodiment 2
[0018] The mass concentration of waste water to be treated is that 30% hydrochloric acid adjusts the pH value to 2, fully stirs for half an hour, the acid consumption is 52Kg, reclaims 58Kg of salicylic acid crystals, and its water content is 16%, records the content of salicylic acid by gas chromatography The purity reaches 98.3%; the recovered salicylic acid crystals are calculated and directly added to the reaction tank as a raw material to carry out the esterification synthesis of the next batch of 2-hydroxybenzoic acid 2-ethylhexyl.
Embodiment 3
[0020] The mass concentration of waste water to be treated is that 30% hydrochloric acid adjusts the pH value to 2, fully stirs for half an hour, the acid consumption is 45Kg, reclaims 55Kg of salicylic acid crystals, and its water content is 15%, records the content of salicylic acid by gas chromatography The purity reaches 98.3%; the recovered salicylic acid crystals are calculated and directly added to the reaction tank as a raw material to carry out the esterification synthesis of the next batch of 2-hydroxybenzoic acid 2-ethylhexyl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com