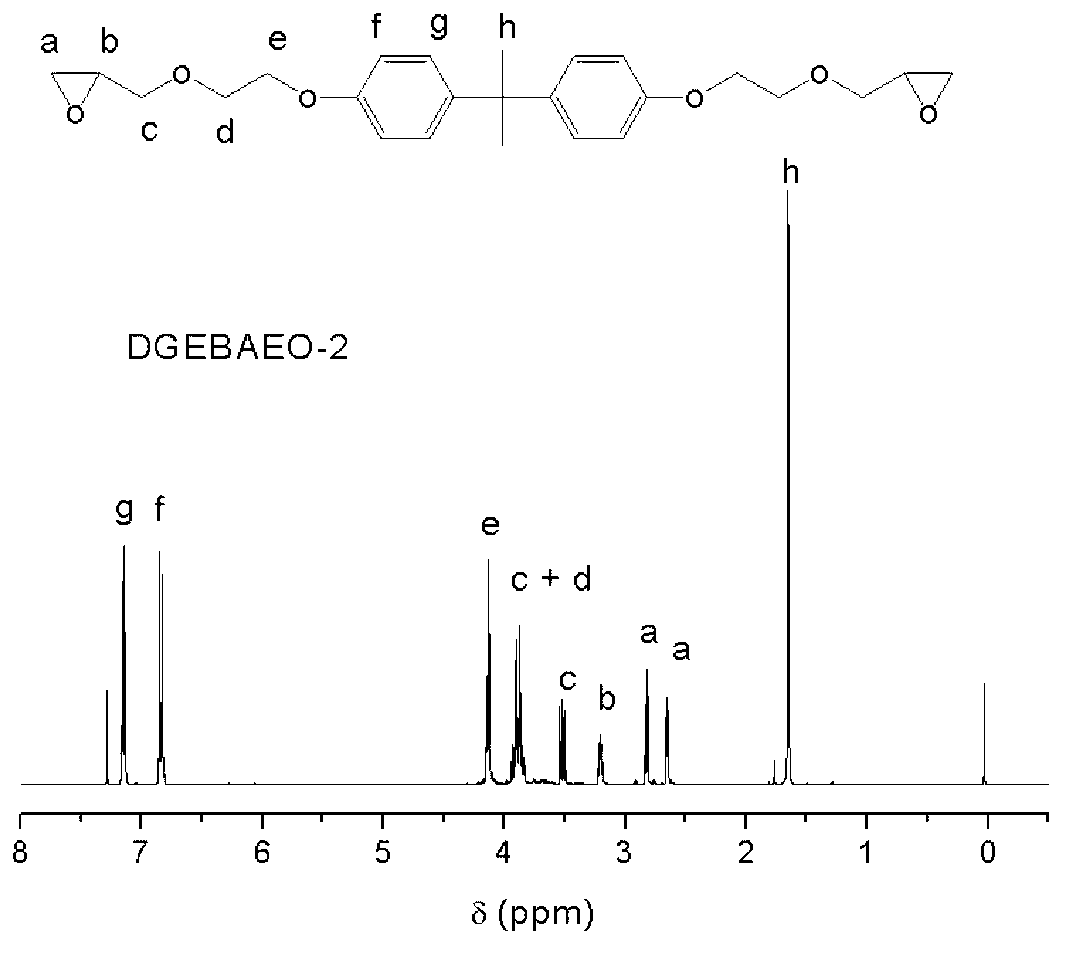
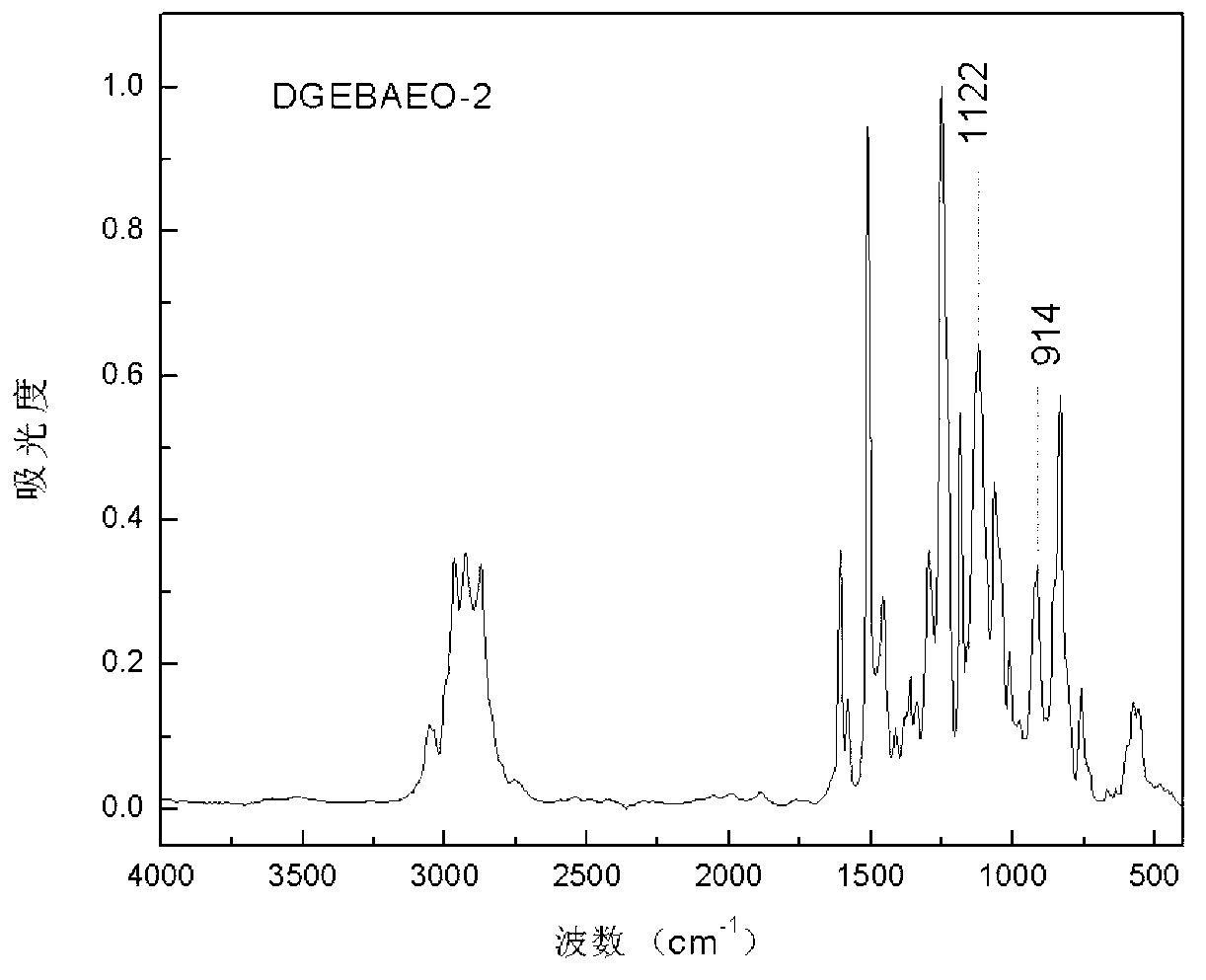
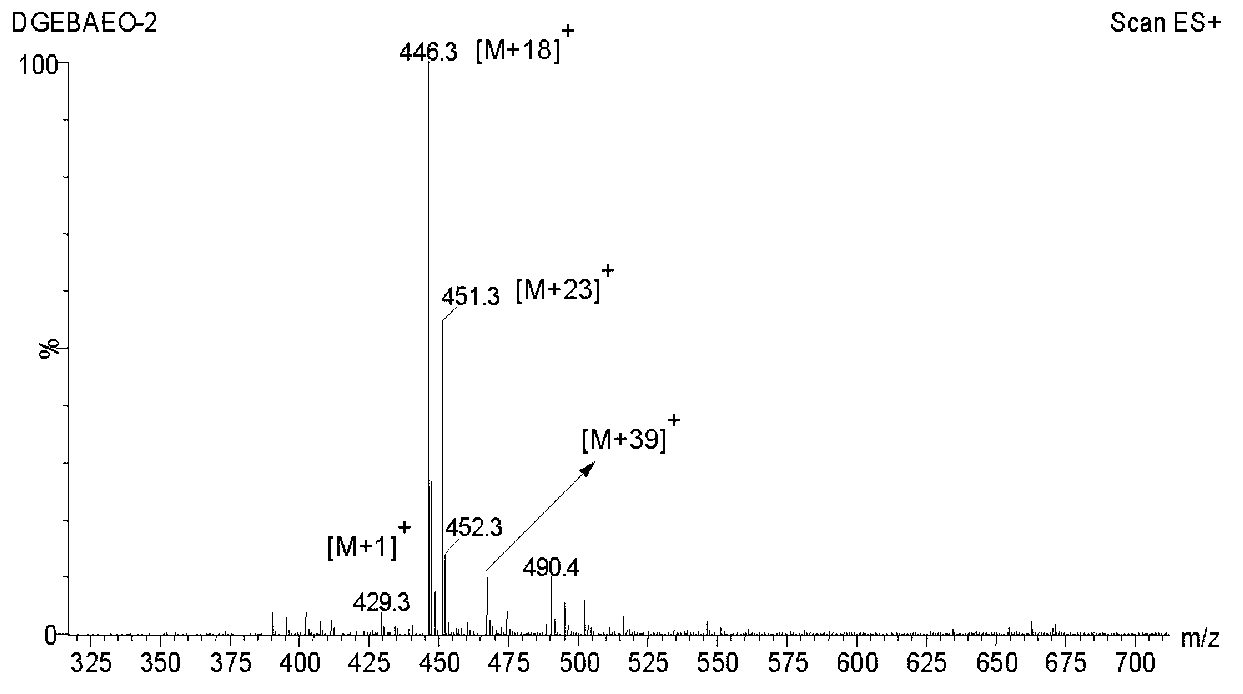Method of preparing glycidyl ether epoxy resin through monomer containing alcoholic hydroxyl group and/or phenolic hydroxyl group
A glycidyl ether and epoxy resin technology, applied in the epoxy resin field, can solve the problems of long reaction time and complicated steps, and achieve the effects of fast reaction rate, high epoxy value and mild reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In a 250mL four-neck flask equipped with a stirring paddle, a thermometer, a condenser, and a nitrogen conduit, add 31.2g of bisphenol A polyoxyethylene ether (BAEO-2) with the following structure and 95g of epichlorohydrin. The system was heated up to 56°C and stirred to fully dissolve the raw materials in epichlorohydrin, then 0.25g of tetramethylammonium bromide and 14g of granular solid sodium hydroxide were added to start timing. During the reaction, the temperature of the system will rise. At this time, use a cold water bath to maintain the reaction system at 56±2°C. When the temperature begins to drop, heat and keep the system at 56±2°C again. After 2.5 hours, the reaction was stopped, and the salt and unreacted base generated by the reaction were removed by suction filtration. Pour the filtrate into a 500mL separatory funnel, add about 130mL of toluene to dilute, and wash the product with deionized water until neutral, then separate the organic phase. Under the...
Embodiment 2
[0042] In a 250mL four-necked flask equipped with a stirring paddle, a thermometer, a condenser, and a nitrogen conduit, 36.6g of bisphenol A polyoxyethylene ether BAEO-2 and 51.2g of epichlorohydrin were added. The system was heated up to 56° C. and stirred to fully dissolve the raw materials in epichlorohydrin, and then 0.3 g of tetramethylammonium bromide and 16.5 g of granular solid sodium hydroxide were added to start timing. Adopt the method of embodiment 1 to control reaction temperature to be 56 ± 2 ℃. After 2.5 hours, the reaction was stopped, and the salt and unreacted base generated by the reaction were removed by suction filtration. Pour the filtrate into a 500mL separatory funnel, add about 150mL of toluene to dilute, and wash the product with deionized water until neutral, then separate the organic phase. Under the pressure of 1-10mmHg, the volatile matter in the organic phase is distilled out by stepwise temperature rise from 60°C to 135°C, and the epoxy value ...
Embodiment 3
[0044]In a 250mL four-necked flask equipped with a stirring paddle, a thermometer, a condenser, and a nitrogen conduit, 31.5g of bisphenol A polyoxyethylene ether BAEO-2 and 94.5g of epichlorohydrin were added. The system was heated up to 56°C and stirred to fully dissolve the raw materials in epichlorohydrin, then 0.26g of tetramethylammonium bromide and 9.5g of granular solid sodium hydroxide were added to start timing. Adopt the method of embodiment 1 to control reaction temperature to be 56 ± 2 ℃. After 2.5 hours, the reaction was stopped, and the salt and unreacted base generated by the reaction were removed by suction filtration. Pour the filtrate into a 500mL separatory funnel, add about 130mL of toluene to dilute, and wash the product with deionized water until neutral, then separate the organic phase. Under the pressure of 1-10mmHg, the volatile matter in the organic phase is distilled out by stepwise temperature rise from 60°C to 135°C, and the epoxy value of the ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com