Multifunctional bioreactor system and methods for cell sorting and culturing
A bioreactor, cell culture technology, applied in the field of multifunctional bioreactors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
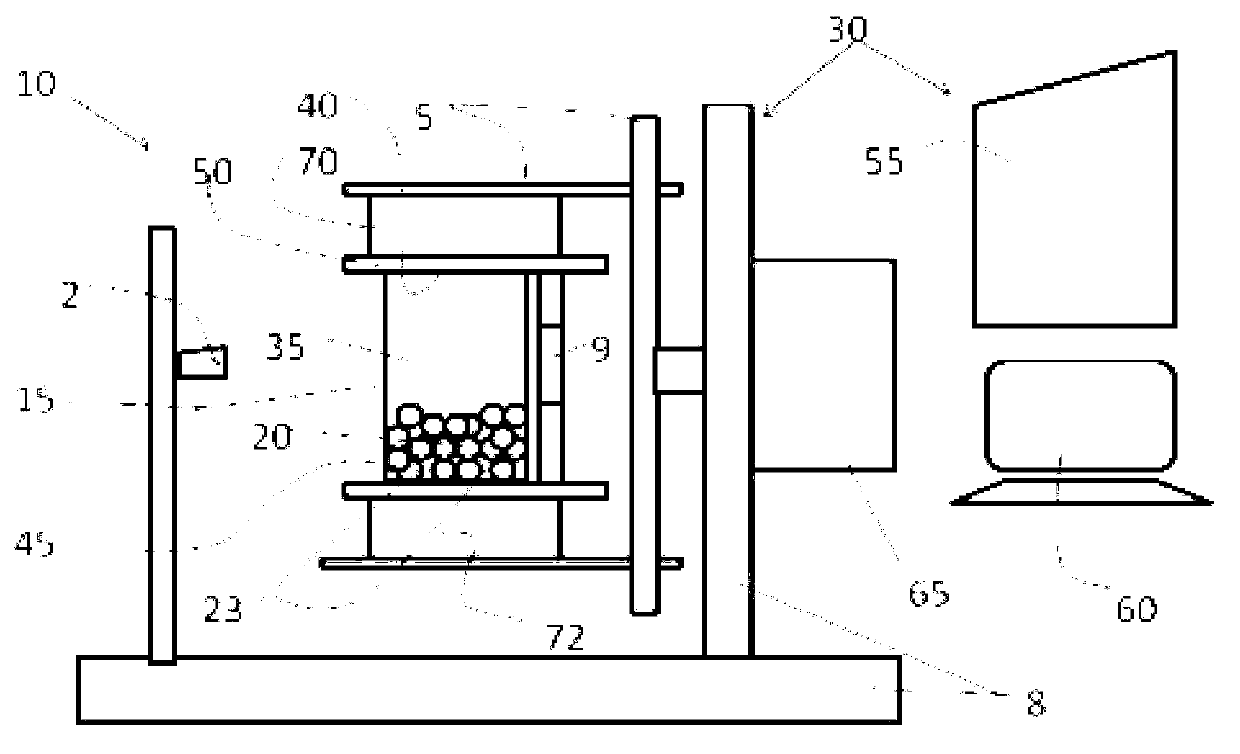
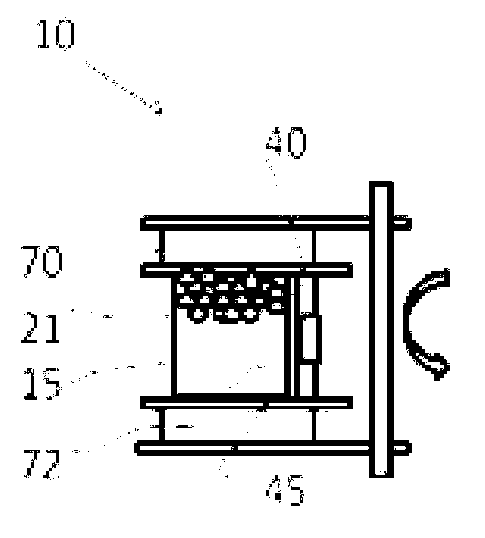
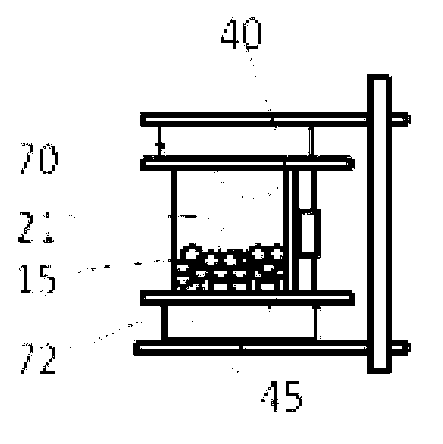
[0041] Example 1. Hematopoietic stem cells (HSCs) were sorted from Buffy's layer cells and then cultured. Parenchyma cells can be obtained from cord blood, bone marrow, peripheral blood or other tissues using standard Ficoll grading procedures. Use conventional methods to count parenchymal cells and resuspend them to the correct volume with the required buffer (e.g. 1% human albumin in PBS), then add the correct amount of magnetically labeled anti-CD34, mix thoroughly, and Incubate at 4°C for 10 to 30 minutes. During the cultivation period, connect the operating system of the bioreactor to the carbon dioxide incubator, connect all the wiring of the control box, and connect to the computer. Power on the bioreactor and computer, select the cell sorting and cell culture program. Depending on cell requirements, edit the program for each specific step or select a general program. After culturing, the cells are placed in a culture chamber containing magnetic beads. can use Fi...
example 2
[0043] Example 2. Expansion of CMV-specific CD8+ cytotoxic T cells. Parenchyma cells obtained from CMV antibody-positive individuals. The application can use 2 methods. The first method is roughly the same as shown in Example 1, but uses magnetically labeled anti-CD8 and CMV-specific CD8+ cytotoxic T cell expansion medium instead of magnetically labeled anti-CD34 and HSC expansion medium , to increase the removal of damaged cells at the end of the culture (see description of the second method below). When cell numbers are low, eg less than one million, the second method is preferred and CD8+ cells are isolated in the middle of the expansion procedure rather than at the beginning. On day 0, after Ficoll isolation, parenchyma cells were washed twice with CTL Medium and resuspended in CTL Medium containing IL-2 and IL-7 to 10 7 / ml, add 10ul CMV pp65 / 10 at 37°C 7 cells / ml PBMC for 5-10 minutes. Cells were then diluted to 10 with stimulation medium 6 / ml, and then placed in...
example 3
[0044] Example 3. Fibroblast culture. Fibroblasts are a typical adherent cell.
[0045] After trypsinization and washing, fibroblasts are resuspended in a suitable medium, such as 10% BSA RPMI 1640, at 10000-40000 / ml. Pour the cells into a cell culture chamber, e.g. Figure 4 The cell culture bag is shown. Connect the culture chamber port with the pipeline, and install the cassette and the culture bag into the flipping arm of the carbon dioxide incubator, as shown in Example 1. Initiate standard adherent cell culture procedures. During this procedure, the cell culture chamber is kept agitated for 24 hours to allow the cells to adhere to the magnetic beads. On the following day, the medium containing non-adherent cells was drained into a waste container or syringe, and fresh medium was added. Cell culture chambers are grown by turning the cell culture chamber slowly and intermittently, followed by programmed media changes during the turning period. Cell culture was stopp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com