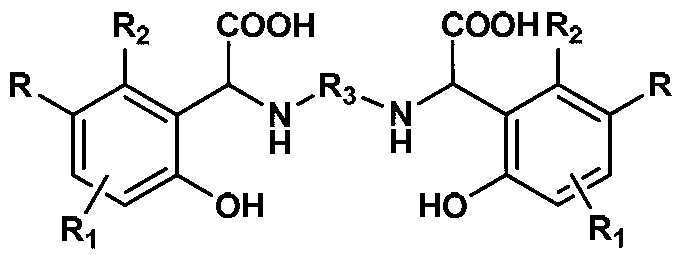
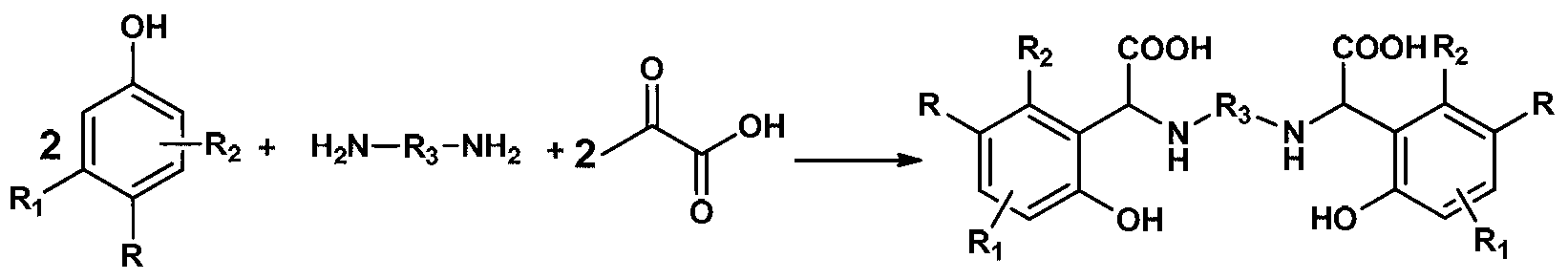
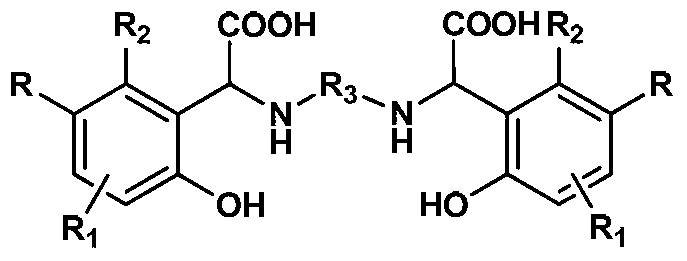
Chelating agent with high stability and high iron chelating ability and preparation method thereof
A high stability, chelating agent technology, applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation, etc., can solve the problems of low yield ortho-body, poor selectivity, excessive phenol raw materials, etc., to achieve high iron Chelating ability, high stability and effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 10.81g (100mmol) p-cresol to a round bottom flask equipped with a condenser tube and a mechanical stirring paddle, heat to 45°C to 50°C to melt it; then add 3.0g (50mmol) ethylenediamine and 0.3g Tetrabutylammonium Hydroxide, stir 10min; Add 13.3g (100mmol) of 30% sodium hydroxide and 17.61g (100mmol) of 50% glyoxylic acid aqueous solution slowly to the above-mentioned mixture again after mixing, be warming up to After reacting at 70°C for 3 hours, cool to room temperature; add 100mL water and 150mL dichloromethane to the above reactants respectively, stir vigorously for 30min, separate the organic phase, and extract the aqueous phase with dichloromethane 3 times, 60ml each time; then water Adjust the pH to 5-6 with 5% HCl, filter and dry at room temperature for about 12 hours to obtain about 17.5 g of white solid, which is the finished product of the chelating agent N,N-m-methyl-o-hydroxyphenylacetoxyethylenediamine. Yield: 90%, Purity: >98% (HPLC).
Embodiment 2
[0022] Add 12.86g (100mmol) p-chlorophenol into a round bottom flask equipped with a condenser tube and a mechanical stirring paddle, heat it to 45°C to 50°C to melt it; then add 3.0g (50mmol) ethylenediamine to the above system and tetrabutylammonium hydroxide 0.3g, stirred for 10min; after mixing evenly, slowly added 13.3g (100mmol) of 30% sodium hydroxide and 17.61g (100mmol) of glyoxylic acid (100mmol) aqueous solution of 50% to the above mixture, and the temperature was raised. After reacting at 75°C for 3h, cool to room temperature; add water (100mL) and dichloromethane (150mL) to the above reactant respectively, stir vigorously for 10min, separate the organic phase, and extract the aqueous phase with dichloromethane three times, each time 60ml. Then the pH of the water phase was adjusted to 5-6 with 5% HCl, and after standing at room temperature for about 12 hours, it was filtered and dried to obtain about 18.67 g of a white solid, which was the product of the chelating...
Embodiment 3
[0024] Add 10.81g (100mmol) p-cresol to a round bottom flask equipped with a condenser tube and a mechanical stirring paddle, heat to 40°C to 45°C to melt it; then add 4.41g (50mmol)1,4 to the above system -Butanediamine and dodecyltrimethylammonium hydroxide 0.5g, stirred for 10min; after mixing evenly, slowly add 30% sodium hydroxide 13.3g (100mmol) and 50% ethyl alcohol dropwise to the above mixture successively 17.61g (100mmol) of aldehyde acid aqueous solution, heated up to 70°C for 3h, then cooled to room temperature; added water (100mL) and dichloromethane (150mL) to the above reactants, stirred vigorously for 10min, separated the organic phase and the aqueous phase Extract with dichloromethane 3 times, 60ml each time, then adjust the pH of the aqueous phase to 5-6 with 5% HCl, leave it at room temperature for about 12 hours, filter and dry to obtain about 17.7g of white solid, which is the chelating agent N,N- The finished product of m-methyl o-hydroxyphenylacetic acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com