Laccase gene, engineering bacteria and application
A technology of genetically engineered bacteria and engineered bacteria, applied in the field of environmental biology, can solve the problems of discovering endogenous laccase and achieve the effect of improving expression activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Optimization of the codon of laccase gene
[0022] According to the cotA gene sequence of Bacillus subtilis (GENE ID: 936023), combined with the codon preference expressed by Pichia pastoris, codon optimization was carried out on the cotA gene sequence, and a restriction site was introduced in the upstream and downstream of the full-length gene: EcoRI and NotI. At the same time, in order to prevent the optimized gene sequence from containing the EcoRI restriction site and the subsequent DNA linearization Bgl II restriction site, and adjust the GC content of the optimized gene, the 855th base A was mutated to G, and the 1098th base was mutated. A base T is mutated to a C. The full length of the gene is 1542 bases, encoding 514 amino acids. The optimized laccase gene was named Tcot.
[0023] Design restriction sites and protective bases at both ends of the gene, and design primers as follows:
[0024] P1: ACGGAATTCATGACTTTGGAAAAGTTTGTTGATGCTTTGCCAATTCCAGATACTTTGAAGCCA...
Embodiment 2
[0056] Expression of Laccase Gene in Yeast Cells
[0057] 1. Construction of expression vector
[0058] The codon-optimized PCR product was digested with EcoRI / NotⅠ, the fragment was recovered, and inserted into the Pichia pastoris constitutive expression vector pGAPZaA (Invitrogen, USA) with the correct reading frame, ligated and transformed E. coli DH5α, the recombinant plasmid pGAPZaA-Tcot was obtained. The recombinant plasmid was verified by EcoRI / NotI double enzyme digestion.
[0059] 2. Preparation of competent cells for electroporation
[0060] In a 50 ml centrifuge tube containing 5 ml YPD, culture yeast cells for 30 overnight; take 30~50 ul of the overnight culture, inoculate a 250 ml shake flask containing 50 ml of fresh medium, grow overnight until OD600=1.3~1.5 ;Collect the cells by centrifugation at 1500 g at 4°C for 5 min, and suspend the cells with 50 ml of pre-cooled sterile water; centrifuge as above, and suspend the cells with 25 ml of pre-cooled sterile...
Embodiment 3
[0076] Study on the Properties of Laccase
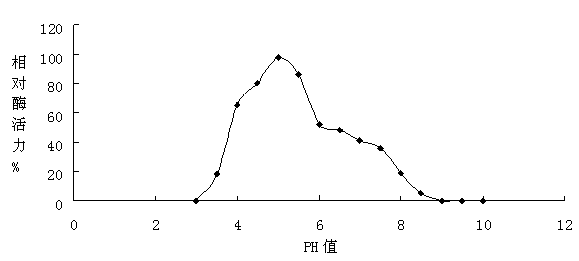
[0077] 1. Effect of pH value on laccase activity
[0078] Laccase was added to the buffer system with different pH values to measure the enzyme activity, the pH was set from 2.0 to 10.0, and points were taken every 0.5 for measurement. The enzyme activity assay reaction was carried out at 25°C, and the reaction time was 15 min. The result is as figure 1 It was shown that the optimum pH value of laccase is 4.5~5.5, and in the range of pH4.0 to pH6.5, the enzyme activity of laccase can maintain more than 50%.
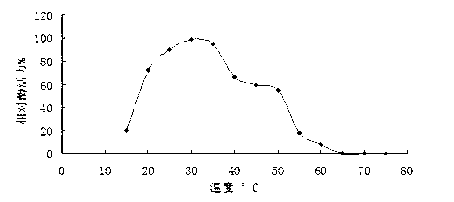
[0079] 2. Effect of temperature on laccase activity
[0080] The optimal reaction temperature of laccase was determined at the optimal pH value, the reaction time was 15 min, the temperature was set from 15 °C to 75 °C, and the measurement was taken every 5 °C. The result is as figure 2 It was shown that the optimum temperature of laccase was 25-35°C, and the enzyme activity could maintain more than 60% at 20-45°C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com