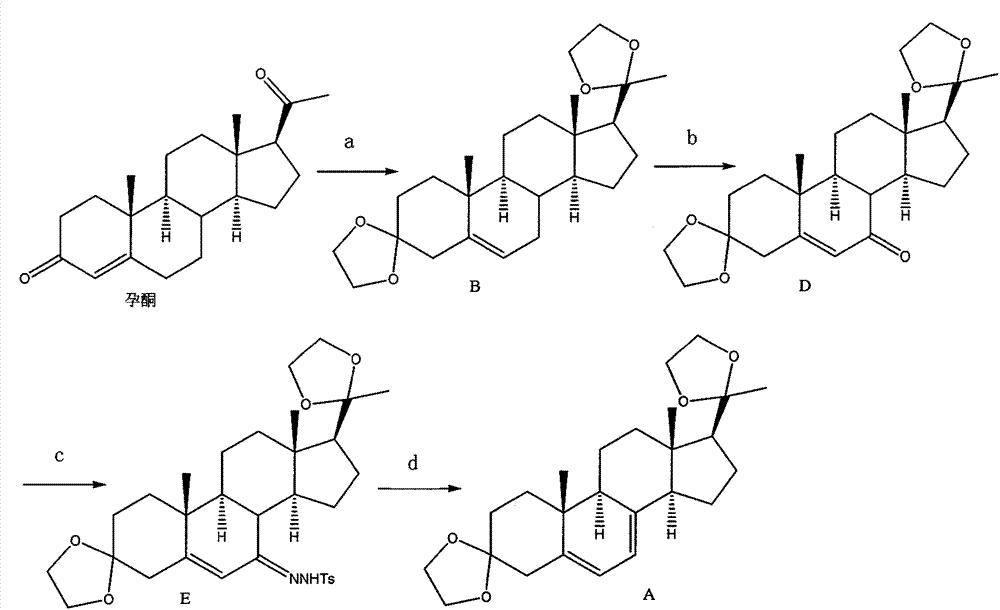
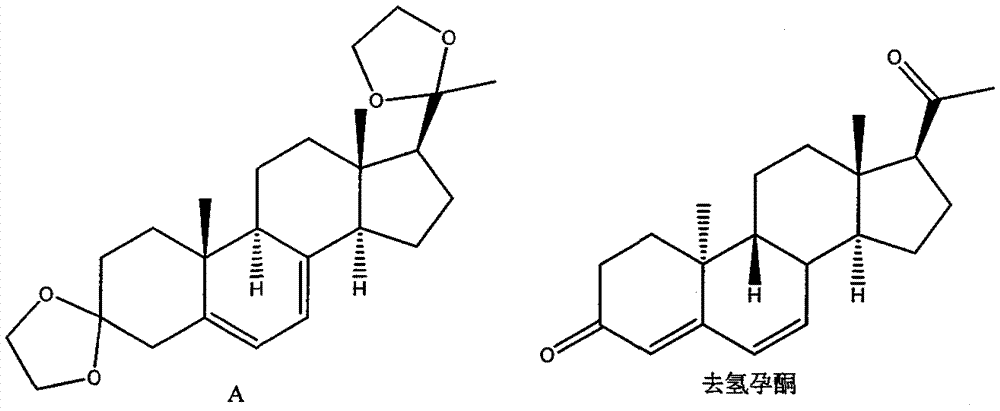
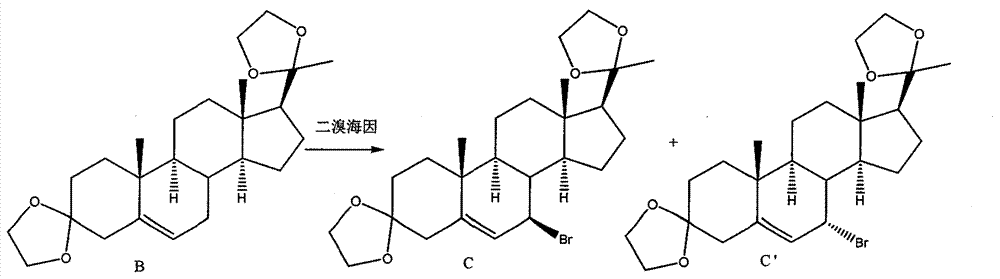
New preparation method of 5,7-pregnadiene-3,20-dione-diethyl ketal
A technology of pregnane and ethylene diketal, which is applied in 5 fields, can solve the problems of tetrabutylammonium fluoride instability, high production and operation costs, and difficulty in long-term storage, and achieve economical and environmentally friendly synthesis routes and good overall recovery. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of compound D
[0028]Put 5.0L of anhydrous ethylene glycol, 3.0g of p-toluenesulfonic acid, and 250g (0.795mol) of progesterone into the reaction bottle. After switching nitrogen three times, turn on the vacuum and start heating, keeping the reaction temperature at 75-80 ℃, while distilling out ethylene glycol. After approximately 3.0L of ethylene glycol has been evaporated, the reaction solution is cooled to room temperature and emptied, and 300ml of ethanol solution with a mass percentage of 20% KOH is added, and stirring is continued for 20 minutes, and 1.7L of dichloromethane is added to dilute the reaction solution, and the layer, the lower layer of dichloromethane was separated, and the upper layer was extracted once with 1.0 L of dichloromethane. Combine the dichloromethane layers, wash the dichloromethane solution with 900ml of water three times, dry over anhydrous sodium sulfate, filter, distill off the dichloromethane under red...
Embodiment 2
[0029] Embodiment 2: the preparation of compound D
[0030] 200g (0.496mol) of compound B, 82g (0.502mol) of N-hydroxyphthalimide, the mixed solvent of 1.6L acetone and 1.6L ethyl acetate were dropped into the reaction flask equipped with stirring device and condenser, and the stirring was started. Raise the temperature to 53±2°C, when the raw materials are completely dissolved, add 1.0g of azobisisoheptanonitrile, and then start to pass in dry purified air 0.8M 3 / hr, after stirring and reacting for 6 hours, TLC tracked that the reaction of the raw materials was substantially complete. Evaporate acetone and ethyl acetate under reduced pressure, add 400ml of dichloromethane, stir for 30 minutes, remove N-hydroxyphthalimide by filtration, wash the filter cake with 200ml of dichloromethane (recovered N-hydroxyphthalimide is reprocessed can be reused later), combined the dichloromethane solution, cooled to 0°C-10°C under nitrogen protection, added 120g (1.18mol) of triethylami...
Embodiment 3
[0032] Embodiment 3: the preparation of compound D
[0033] Put 82g (0.502mol) of N-hydroxyphthalimide, 1.0g of dibenzoyl peroxide, and 1.6L of cyclohexanone into the reaction flask equipped with a stirring device and a condenser, and then heat to 54°C-55°C ℃, stir until the raw materials are completely dissolved, and pass through dry and purified air (0.8M 3 / hr) until 30 minutes later, 200 g (0.496 mol) of compound B was added, air was continuously introduced, and the reaction was stirred for 6 hours, followed by TLC until the reaction of the raw materials was substantially complete. Distill cyclohexanone off under reduced pressure, add 400ml of dichloromethane, stir for 30 minutes, filter to remove N-hydroxyphthalimide, wash the filter cake with 200ml of dichloromethane, combine the dichloromethane solution, and cool to 0 ℃-10℃, add 120g (1.18mol) of triethylamine under stirring, keep the temperature at 0℃-10℃, add 60g (0.588mol) of acetic anhydride dropwise, and stir fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com