Weight reduction and body fat reduction applications of chitosan oligosaccharide
A technology of chitosan oligosaccharide and number average molecular weight, applied in the field of medicine, can solve problems such as influence on the popularization and application of chitosan oligosaccharide, unclear application mechanism, unstable application effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1 Materials and methods
[0033] 1.1 Test samples
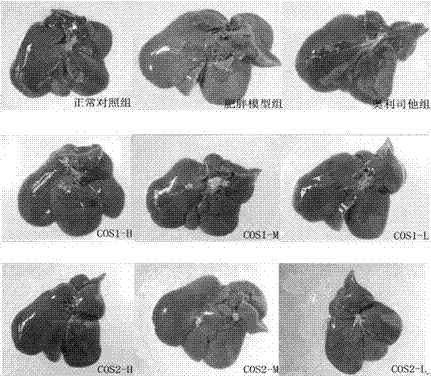
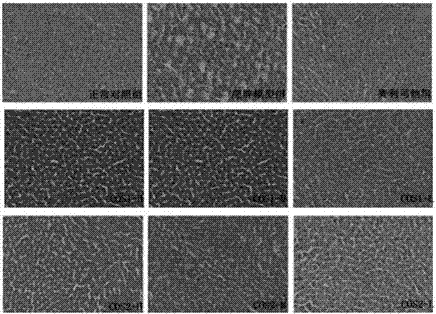
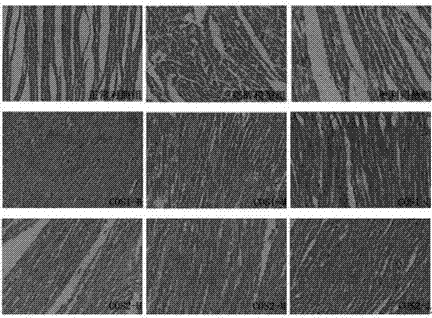
[0034] Chitooligosaccharides (Shandong Laizhou Haili Biological Products Co., Ltd., COS1 number average molecular weight ≤ 1000, production batch number HL120916G; COS2 number average molecular weight ≤ 3000, production batch number HL120911G; COS3 number average molecular weight ≤ 5000, production batch number HL120907G).
[0035] 1.2 Experimental animals
[0036] 185 male Sprague Dawley rats of SPF grade (permit number: SCXK (Guangdong) 2008-0002), weighing 70-90 g, were purchased from Guangdong Medical Experimental Animal Center, and were fed adaptively for one week.
[0037] 1.3 Reagents and Instruments
[0038] Orlistat Capsules (Chongqing Huasen Pharmaceutical Co., Ltd.); Alanine Aminotransferase Assay Kit (ALT), Aspartate Aminotransferase Assay Kit (AST), Triglyceride Assay Kit (TG) , total cholesterol assay kit (CHO), high-density lipoprotein cholesterol assay kit (HDL-C), low-density lipoprotein cho...
Embodiment 2
[0125] Embodiment 2 toxicity test
[0126] 1 Pre-experiment
[0127] SPF-grade NIH mice, weighing 18-22 g, half male and half male, were reared in separate cages, fed adaptively for 3 days in an environment with a temperature of 22±1°C and a relative humidity of 50-60%, with free access to food and drinking water. limit. In the experiment, the maximum concentration of COS1 and COS2 described in Example 1 (the drug just passed through the gavage needle smoothly, and the physical and chemical properties did not change) was used to gavage the mice, and the pre-experiment set up 3 dosage groups, respectively 16g / Kg (Give once), 32g / Kg (Give twice), 48g / Kg (Give three times), each dose group has 10 mice, half male and half male. Before administration, fast for 16 hours, without restriction on drinking water, the volume of each administration is 0.2mL / 10g, and the interval between each administration is 5h.
[0128] COS1 pre-test results:
[0129] 1.16g / Kg dose: the mice had...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com