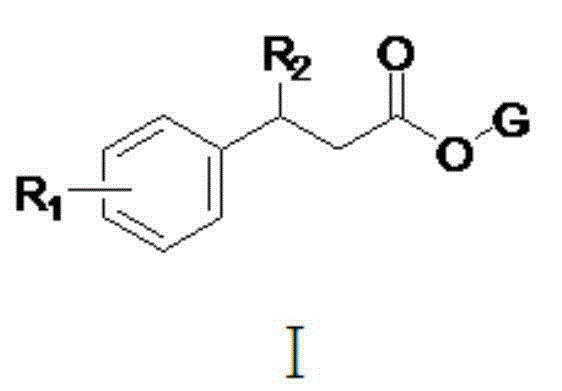
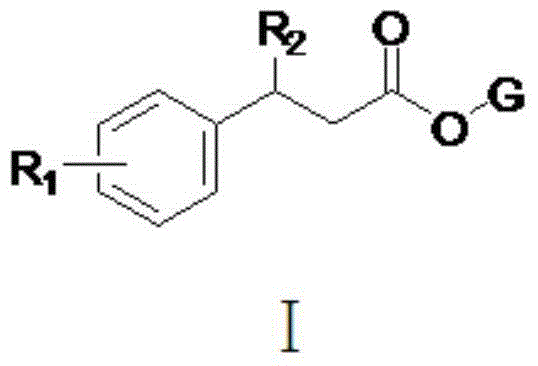
Pyrethroid compound, and preparation method and applications thereof
A technology for pyrethroids and compounds, which is applied in the field of pyrethroid compounds and their preparation, and can solve the problems of low activity and phytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
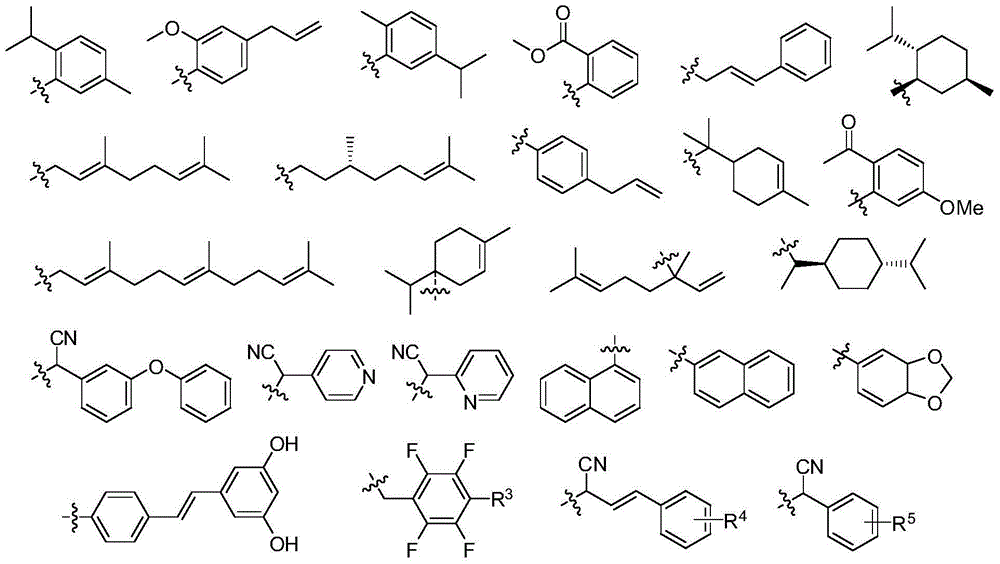
[0028] The structure of some compounds of the present invention is one of the specific compounds listed in Table 1: Table 1 Representative Compound Structure List
[0029]
[0030]
[0031]
[0032]
[0033]
Embodiment 2
[0035] For the preparation of polysubstituted carboxylic acids, take intermediate III-1 as an example.
[0036]
[0037] Add compound II-1 (8mmol) into a 100mL one-necked flask, then add 40mL of tetrahydrofuran and stir to dissolve it, then add 10mL of water, cool the reaction solution to 0°C, add lithium hydroxide (9.6mmol) and hydrogen peroxide under stirring (19.2mmol), reacted at 0°C for 24h, added a small amount of sodium sulfite to remove excess hydrogen peroxide, added dropwise dilute hydrochloric acid to adjust the pH of the solution to 2-3, extracted with ether (2x30mL), washed the organic phase with saturated brine (50mL), washed with water, no Water Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain the crude product III-1, which is directly used in the next reaction.
Embodiment 3
[0039] For the preparation of pyrethroid compounds, take compound I-A1 as an example.
[0040]
[0041] Weigh the crude product III-1 (1g) from the previous step, add thymol 0.31g (2.08mmol), acetonitrile (10mL), stir to dissolve, then add catalytic amount of DMAP (0.1g) and condensing agent DCC0.515g (2.50mmol), stirred at room temperature for 16h, removed DCU by suction filtration, washed the filtrate with aqueous sodium carbonate (2x30mL), extracted with ethyl acetate (2x30mL), combined the organic phase, washed the organic phase with saturated brine (50mL), and washed with water. Anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain a crude product, which is separated by column chromatography to obtain compound I-A1. The basic physical and chemical properties of the target compound I-A1 are as follows: White solid, mp: 53-54°C.IR (KBr):υ3063,3029,2960,2917,2865,1745,1505,1486,1454,1136,816,757,697cm -1 . 1 H NMR (400MHz, CDCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com