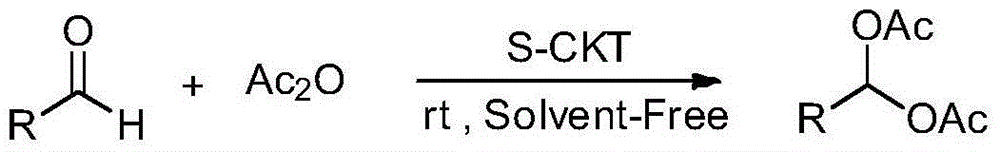
1,1-diacetate synthesis catalyzed by sulfonated cage-type mesoporous carbon
A technology for sulfonating cage type and diacetate, which is applied in the field of carbonyl ester compounds, can solve the problems of difficult separation of products, long reaction time, low recovery rate and the like, and achieves high yield, mild reaction conditions, and high reaction short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
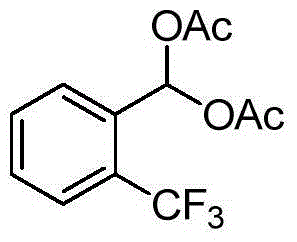
[0023] Taking the synthesis of 1,1-diacetoxy-1-(2-trifluoromethylphenyl)methane as an example, 1,1-diacetoxy-1-(2-trifluoromethylphenyl)methane The structural formula is:
[0024]
[0025] Its synthesis method is as follows:
[0026] Add 2mmol of 2-trifluoromethylbenzaldehyde, 5mmol of acetic anhydride, and 30mg of sulfonated cage-type mesoporous carbon into a 25mL single-necked bottle, stir and react for 5 minutes at room temperature without solvent, track the reaction until complete by TLC, add ethyl acetate 20-30mL, filter the catalyst, and use saturated NaHCO 3 The solution was washed 3 times, washed 1 time with water, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (the volume ratio of ethyl acetate to petroleum ether as the eluent was 1:8) to obtain a pure product with a yield of 96%, m.p.71-72°C; 1 H NMR (4...
Embodiment 2
[0029] Taking the synthesis of 1,1-diacetoxy-1-(2-bromo-4,5-dimethoxyphenyl)methane as an example, 1,1-diacetoxy-1-(2-bromo-4 ,5-dimethoxyphenyl)methane has the structural formula:
[0030]
[0031] Its synthesis method is as follows:
[0032] Add 2mmol of 2-bromo-4,5-dimethoxybenzaldehyde, 5mmol of acetic anhydride, and 30mg of sulfonated cage-type mesoporous carbon into a 25mL single-necked bottle, stir and react at room temperature for 5 minutes without solvent, and track the reaction to complete, add 20-30 mL of ethyl acetate, filter the catalyst, and use saturated NaHCO 3 The solution was washed 3 times, washed 1 time with water, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (the volume ratio of ethyl acetate to petroleum ether as the eluent was 1:5) to obtain a pure product with a yield of 97%, m.p.84-85°C;...
Embodiment 3
[0034] Taking the synthesis of 1,1-diacetoxy-1-(phenyl)methane as an example, the structural formula of 1,1-diacetoxy-1-(phenyl)methane is:
[0035]
[0036] Its synthesis method is as follows:
[0037] Add 2 mmol of benzaldehyde, 5 mmol of acetic anhydride, and 30 mg of sulfonated cage-type mesoporous carbon into a 25 mL single-necked bottle, stir and react at room temperature for 5 minutes without solvent, and track the reaction until complete with TLC. Add 20-30 mL of ethyl acetate, filter the catalyst, and wash the filtrate with saturated NaHCO 3 The solution was washed 3 times, washed 1 time with water, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a crude product. The crude product was recrystallized with ethanol to obtain a pure product, the yield was: 96%, m.p.65-66 ° C, MS (EI): 238 (M + ),179,153,135,107,92,77,43.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com