Preparation method and application of N-substituted hydroxamic acid compound
A technology of a compound and a target compound, applied in the field of preparation and use of N-substituted hydroxamic acid compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
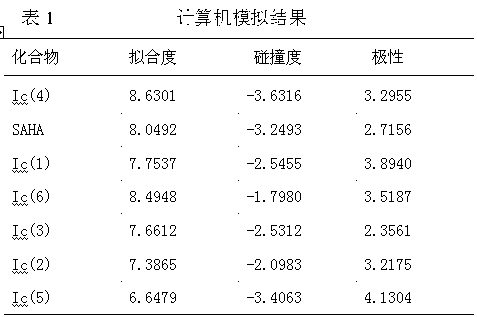
Method used
Image
Examples
Embodiment 1
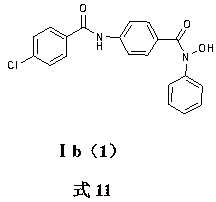
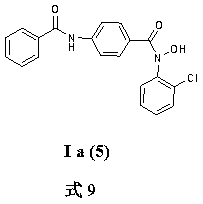
[0049] Example 1 Preparation of N-hydroxyl-N-phenyl-4-benzamido-benzamide I a (1)
[0050] Weigh 3g (20mmol) of methyl 4-aminobenzoate and dissolve it in 40ml of anhydrous tetrahydrofuran, add 30mmol of sodium bicarbonate, add dropwise 20ml of anhydrous tetrahydrofuran solution containing 20mmol of benzoyl chloride, stir at room temperature for 4 hours, filter, and depressurize Distill to obtain white solid 4-benzamidomethyl benzoate; dissolve with 50ml THF, add 15mmol 4-benzamidomethylbenzoate, add 30ml of 1mol / L sodium hydroxide solution, heat to reflux for 5 hours, depressurize Part of the solvent was evaporated, slowly poured into 100ml of an aqueous solution dissolved with 30mmol of hydrochloric acid, filtered and dried to obtain compound 4-benzamidobenzoic acid; 15mmol of compound 4-benzamidobenzoic acid was dissolved in 40ml of anhydrous tetrahydrofuran, and 4 drops of DMF, add 18 mmol of oxalyl chloride dropwise in an ice-water bath, stir at room temperature for 4 hour...
Embodiment 2
[0053] Example 2 Preparation of N-hydroxy-N-o-methylphenyl-4-benzamido-benzamide Ia (2)
[0054] Preparation method: replace the nitrogen phenyl hydroxylamine in Example 1 with N-(2-methylphenyl) hydroxylamine, and the other preparation methods are the same as in Example 1. The product is a white solid, and the structural formula is shown in formula 6,
[0055]
[0056] Yield 37.6%, mp 236.2-238.4?C.; 1 HNMR (400MHz DMSO-d 6 ): δ 10.47(s, 1H), 9.84(s, 1H), 8.06-7.95(m, 4H), 7.87-7.81(m, 2H), 7.64-7.52(m, 4H), 7.36-7.9515(m, 4H), 2.26(s, 3H).; 13 CNMR (100MHz DMSO-d 6 ): Δ 165.7, 164.7, 141.4, 141.0, 136.5, 134.6, 133.6, 131.7, 130.2, 129.6, 129.2, 127.7, 126.5, 125.9, 119.4, 17.9.; , found 347.1381..
Embodiment 3
[0057] Example 3 Preparation of N-hydroxyl-N-m-methylphenyl-4-benzamido-benzamide Ia (3)
[0058] Preparation method: replace the nitrogen phenyl hydroxylamine in Example 1 with N-(3-methylphenyl) hydroxylamine, and the other preparation methods are the same as in Example 1. The product is a white solid, and the structural formula is shown in formula 7,
[0059]
[0060] Yield 44.3%, mp 233.9-235.1?C.; 1 HNMR (400MHz DMSO-d 6 ): δ 10.67(s, 1H), 10.46(s, 1H), 7.97(d, J = 7.2 Hz, 2H), 7.84(d, J = 8.4Hz, 2H), 7.69-7.53(m, 5H), 7.40(s, 1H), 7.34-7.24(m, 2H), 7.02(d, J = 7.2 Hz, 1H), 2.32(s, 3H).; 13 CNMR (100MHz DMSO-d 6 ): Δ 167.4, 165.8, 142.3, 141.0, 137.8, 134.7, 131.8, 130.2, 129.4, 127.9, 127.6, 126.1, 119.5, 119.2, 21.1.; , found 347.1383..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com