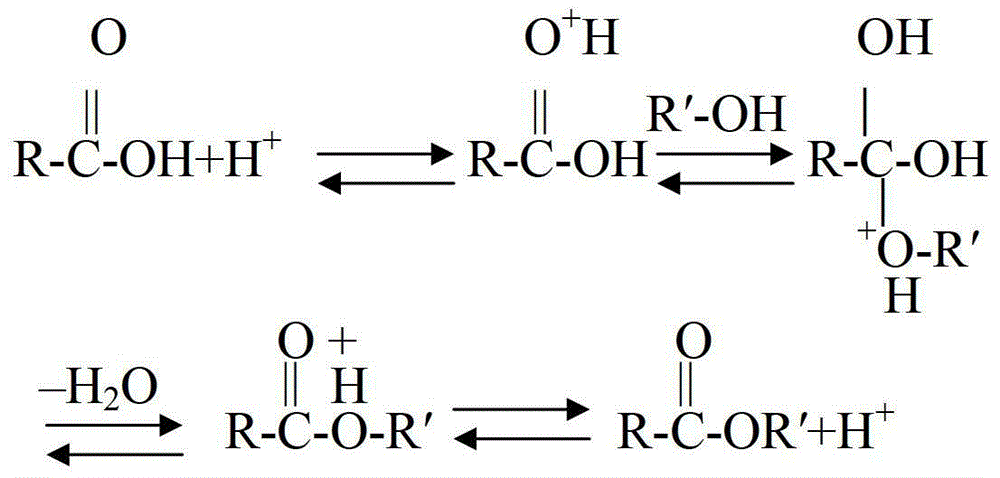
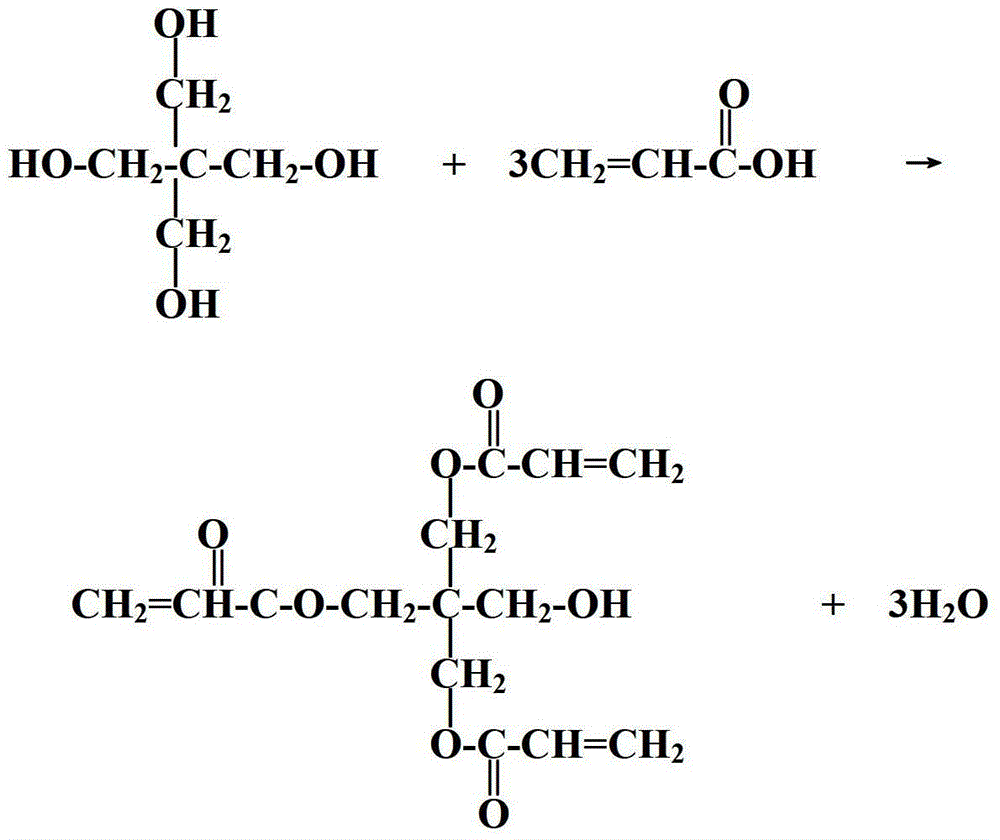Method for preparing pentaerythritol triacrylate
A technology of pentaerythritol triacrylate and pentaerythritol, which is applied in the field of compound synthesis, can solve the problems of low product yield, explosion hazard, easy pollution of waste water environment, etc., achieve high product yield and improve product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of pentaerythritol triacrylate includes the following steps:
[0031] 1) Put 75g of pentaerythritol, 131g of acrylic acid, 4.5g of solid acid catalyst, and 0.5g of phenothiazine in a 500ml three-necked flask, turn on the stirring, stir and react for 2 hours to carry out the esterification reaction.
[0032] 2) Turn on the vacuum pump, control the pressure in the reaction flask to 0.05Mpa, then raise the temperature in the flask to 120°C to cause reflux, and continuously flow out the water produced by the reaction through the water separator. The reaction time is 5 hours, and the reaction is detected The acid value of the solution was 30 mgKOH / g, the temperature was stopped, and the temperature began to drop to room temperature.
[0033] 3) Filter the material, distill off the residual acrylic acid and water under the conditions of 98°C and 0.09Mpa vacuum. When the acid value is controlled at 0.92mgKOH / g, the distillation can be stopped and the temperature ...
Embodiment 2
[0035] A preparation method of pentaerythritol triacrylate includes the following steps:
[0036] 1) 75g of pentaerythritol, 131g of acrylic acid, 4.5g of solid acid catalyst, 0.5g of p-hydroxyanisole, placed in a 500ml three-necked flask, turned on the stirring, stirred and reacted for 2 hours to proceed the esterification reaction.
[0037] 2) Turn on the vacuum pump, control the pressure in the reaction flask to 0.075Mpa, and then increase the temperature in the flask to 110°C to cause reflux, and the water produced by the reaction will flow out continuously through the water trap. The reaction time is 3.5 hours, and the reaction is detected The acid value of the solution is 40 mgKOH / g, and the temperature rise is stopped. Start to cool down to room temperature.
[0038] 3) Filter the material, distill off the residual acrylic acid and water under the conditions of temperature 105℃ and vacuum degree of 0.095Mpa. When the acid value is controlled at 0.76mgKOH / g, the distillation c...
Embodiment 3
[0040] A preparation method of pentaerythritol triacrylate includes the following steps:
[0041] 1) Put 75g of pentaerythritol, 131g of acrylic acid, 4.5g of solid acid catalyst, and 0.5g of p-hydroxyanisole into a 500ml three-necked flask, turn on the stirring, stir and react for 2 hours to carry out the esterification reaction.
[0042] 2) Turn on the vacuum pump, control the pressure in the reaction flask to 0.098Mpa, then raise the temperature in the flask to 98°C to cause reflux, and continuously flow out the water produced by the reaction through the water separator. The reaction time is 2 hours, and the reaction is detected The acid value of the solution is 50 mgKOH / g, and the temperature rise is stopped. Start to cool down to room temperature.
[0043] 3) Filter the material, distill off the residual acrylic acid and water under the conditions of temperature 115℃ and vacuum degree 0.098Mpa. When the acid value is controlled at 0.58mgKOH / g, the distillation can be stopped an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com