Quality control method of traditional Chinese medicine tablet for treating nasosinusitis
A quality control method and technology of traditional Chinese medicine tablets, which are applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as being unsuitable for diabetics, unable to guarantee the quality of finished products, and complicated in operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
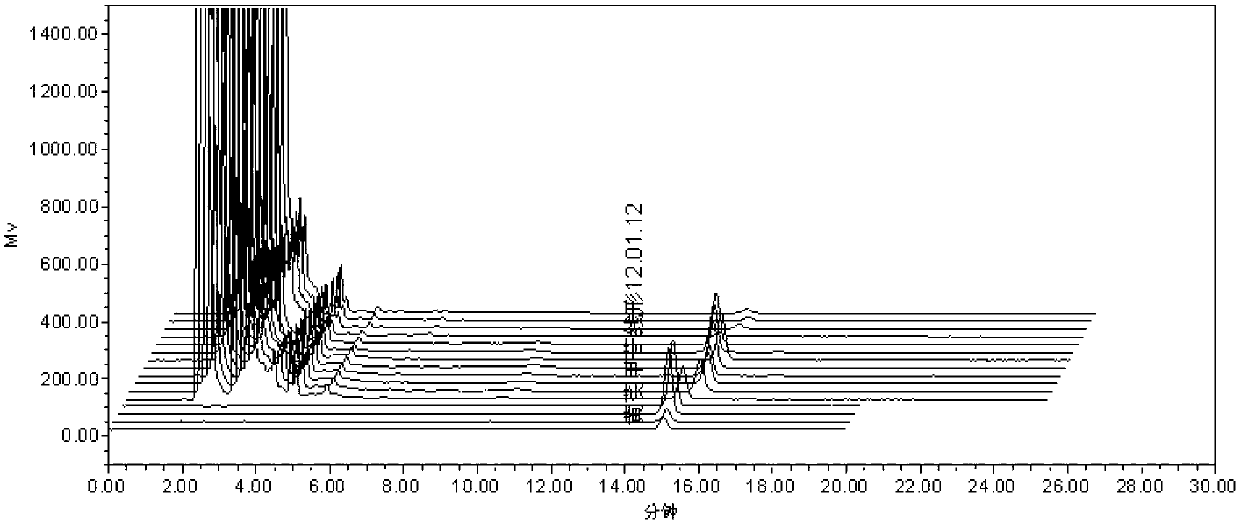
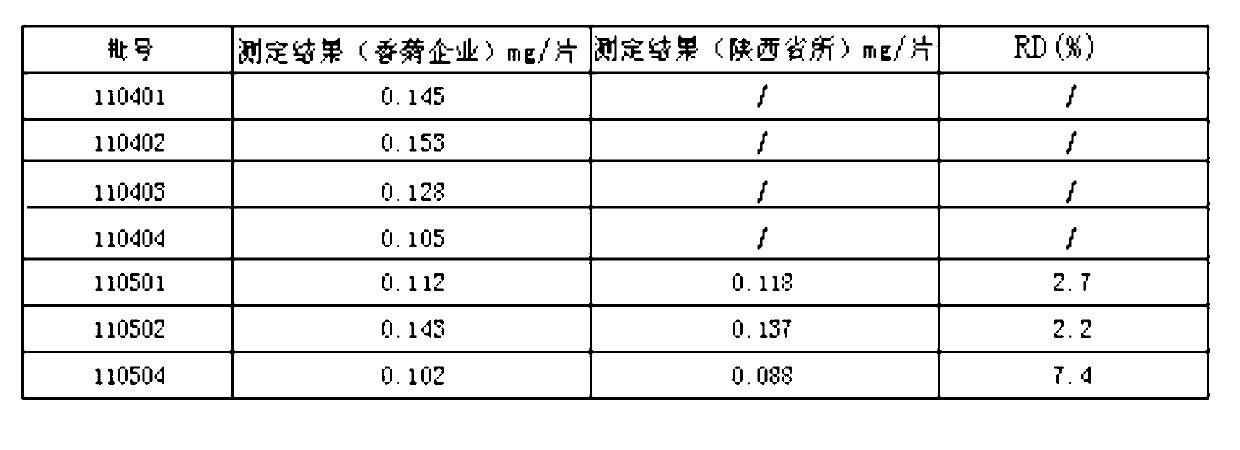
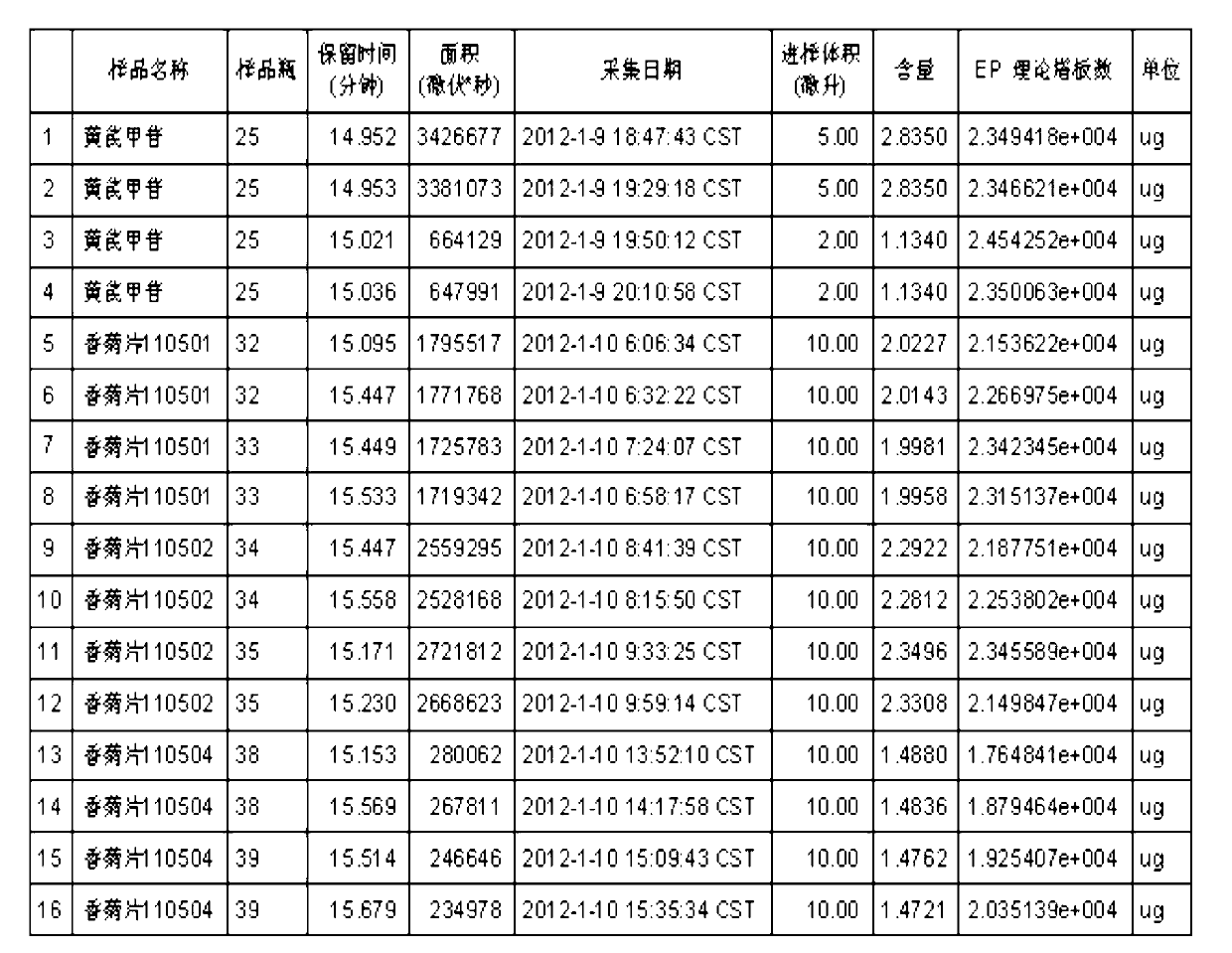
[0038] (1) TLC identification method of gallic acid, an index component of the fruit order of A.
[0039] for new items. The fruit sequence of Fructus fragrans is the main drug in the prescription, which contains compounds such as tannins and flavonoids. Using gallic acid as a control, the identification method of TLC was studied.
[0040] Preparation of test solution: take 2 pieces of sample, grind finely, add 30 ml of dilute ethanol, ultrasonically treat for 15 minutes, filter, steam the filtrate in a water bath until there is no alcohol smell, put it at room temperature, add hydrochloric acid to the filtrate to adjust the pH to 2~3, use Diethyl ether was shaken and extracted twice, 20 ml each time, the extracts were combined, evaporated to dryness, the residue was dissolved in methanol to 1 ml, and used as the test solution.
[0041] Preparation of reference substance solution: take an appropriate amount of gallic acid reference substance, add absolute ethanol to make a so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com