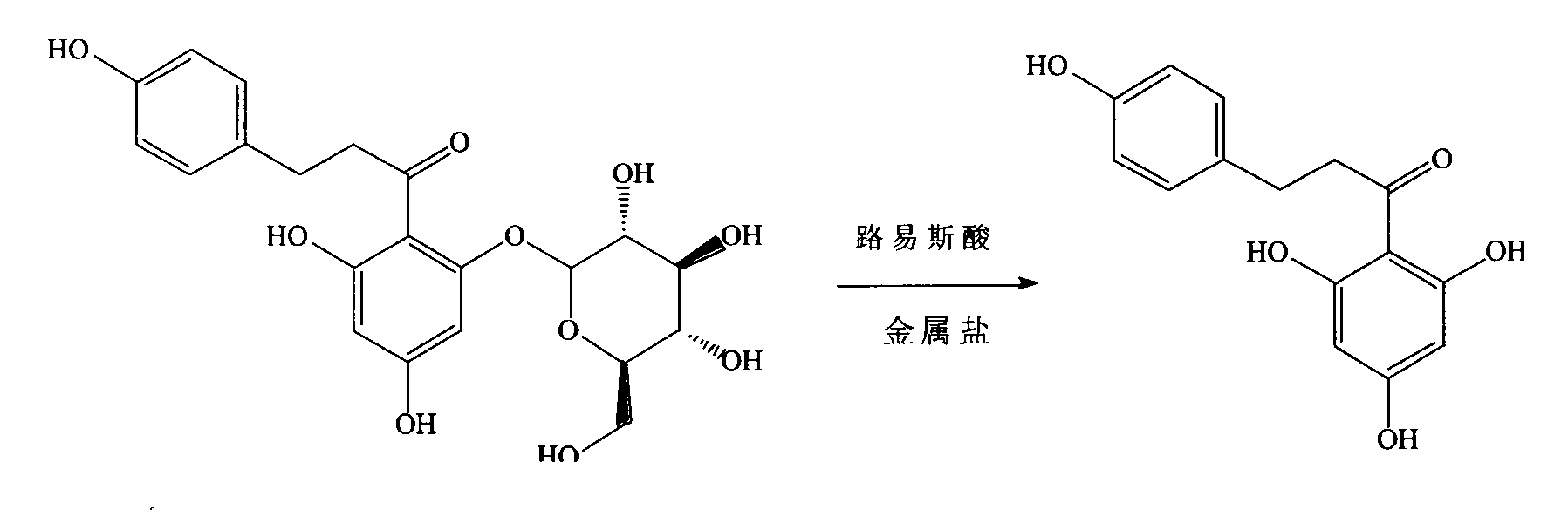
Technology for semisynthesis of phloretin from natural phlorizin
A technology of phloretin and phloretin, which is applied in the field of semi-synthesis of phloretin, can solve the problems of limited market supply of phloretin, high price, and low phloretin content, and achieve green production and controllable process , The effect of simple reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of embodiment 1 phloretin
[0023] 50.0kg of crude phloridin (the content of phloridin is about 90%), in a 500L stainless steel reaction kettle, add about 300 liters of 5% sodium hydroxide solution, stir at room temperature to dissolve phloridin, after about 1h, vacuum filter , The filtrate was transferred to another 500L enamel reaction kettle. Stir and put ice salt water in the jacket to cool down. When the temperature in the reactor drops below 5°C, slowly add about 1.9L of boron trifluoride ether solution, the pH value is about 1.5, and the process control temperature is not higher than 10°C . Then continue to add 0.6 kg of nickel chloride hexahydrate at one time, and gradually raise the temperature to 35° C., stir and react for 12 hours, monitor the disappearance of phlorizin spots by thin-layer chromatography, and consider the reaction as complete. After the reaction was completed, the temperature was lowered to 0°C, and the stirring was continued,...
Embodiment 2
[0024] The synthesis of embodiment 2 phloretin
[0025] 50.0kg of crude phloridin (the content of phloridin is about 90%), in a 500L stainless steel reaction kettle, add about 300 liters of 5% sodium hydroxide solution, stir at room temperature to dissolve phloridin, after about 1h, vacuum filter , The filtrate was transferred to another 500L enamel reaction kettle. Stir and cool the jacket with ice-salt water. When the temperature in the reactor drops below 5°C, slowly add about 1.6L of phosphorus tribromide, the pH value is about 1, and the process control temperature is not higher than 10°C. Then continue to add 0.4 kg of ferric chloride at one time, and gradually raise the temperature to 35° C., stir and react for 15 hours, monitor the disappearance of phlorizin spots by thin-layer chromatography, and consider the reaction as complete. After the reaction was completed, the temperature was lowered to 0°C, and the stirring was continued, and phloretin crystals were graduall...
Embodiment 3
[0026] Embodiment 3 Phloretin is refined
[0027] Heat and dissolve 40.0kg of phloretin coarse crystals with 400L of ethanol solution containing 95% content, pass through a fine filter to a 500L stainless steel crystallization tank, concentrate under reduced pressure to obtain 300L of ethanol, and then cool to 10°C to crystallize while stirring, and then crystallize after 2 hours Suffering. Then double-cone drying was used for 2 hours to obtain 31.0 kg of fine benzfluorenol, the content and purity of which were greater than 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com