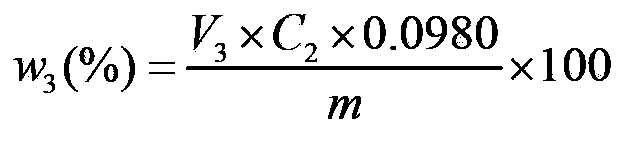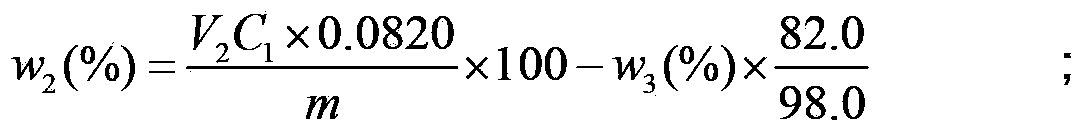Method for determination of the content of main components of phosphorus trichloride hydrolysis solution
A technology of phosphorus trichloride and determination method, which is applied in the direction of chemical analysis by titration method, can solve the problems such as inability to obtain content data, inability to measure phosphoric acid content, inability to accurately determine the content of hydrochloric acid, etc., and achieves good reproducibility, High accuracy and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Accurately weigh 1.2085 g of phosphorus trichloride hydrolyzate sample with a weighing bottle, add it into a 250 mL beaker filled with 150 mL of water, place it on a magnetic stirrer, insert a precision pH meter electrode, start stirring, and use a concentration of 0.5042 mol / L of sodium hydroxide standard titration solution is dripped to solution pH=4.5, and the volume V of the consumed sodium hydroxide standard titration solution is recorded 1 It is 20.23mL; continue to titrate with sodium hydroxide standard titration solution with a concentration of 0.5042mol / L until pH=9.5, and record the volume V of the standard titration solution consumed 2 It is 5.71mL; then add 10mL of neutral calcium chloride solution with a concentration of 100g / L adjusted to reddish with 0.05mol / L sodium hydroxide solution before using phenolphthalein as indicator, and use a 10mL alkaline burette Fill 0.05mol / L sodium hydroxide standard titration solution, drop to pH=7.53, write down the vol...
Embodiment 2
[0059] Embodiment 2 (standard addition recovery test)
[0060] In order to further verify the accuracy of the results, a standard recovery test was also carried out in this example:
[0061] 1) Take an appropriate amount of analytically pure hydrochloric acid, phosphoric acid, and phosphorous acid, and prepare them into hydrochloric acid solution, 20% phosphorous acid solution, and 0.5% phosphoric acid solution with a mass fraction of 20%, respectively, and measure their concentrations according to the methods stipulated in the national standard. exact content. The content of hydrochloric acid (HCl) in hydrochloric acid solution was 20.12%, the content of phosphorous acid in phosphorous acid solution was 19.88%, and the content of phosphoric acid in phosphoric acid solution was 0.56%.
[0062] 2) Use the phosphorous trichloride hydrolyzate with known contents of hydrochloric acid, phosphorous acid and phosphoric acid in the above Example 1 as the sample in a weighing bottle, ...
Embodiment 3
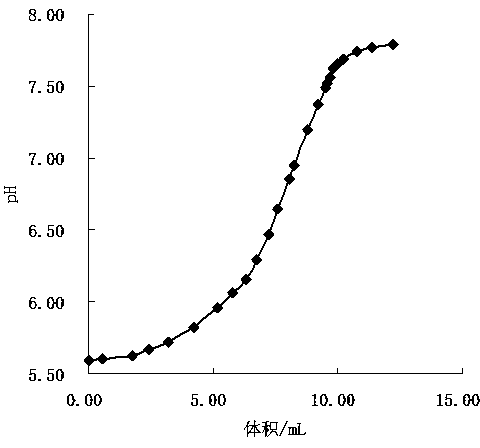
[0074] In order to further accurately determine the main components of the phosphorus trichloride hydrolyzate, the present embodiment also takes the phosphoric acid solution of known concentration as a sample, and measures according to the method of the present invention. When adjusting its pH value to 9.5, add neutral chloride Calcium solution, titrate with 0.05mol / L sodium hydroxide standard titration solution, record the change of pH value with the volume of titrant during the titration process, and determine the pH value of the stoichiometric point at which disodium hydrogen phosphate is completely titrated.
[0075] Accurately take by weighing content to be the analysis pure phosphoric acid 0.0585g of 85.0%, after adding neutral calcium chloride solution in the step in embodiment 1, titrate with the sodium hydroxide standard titration solution of 0.05255mol / L, record pH in the titration process The value varies with the volume of the titrant. Take the consumed volume of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com