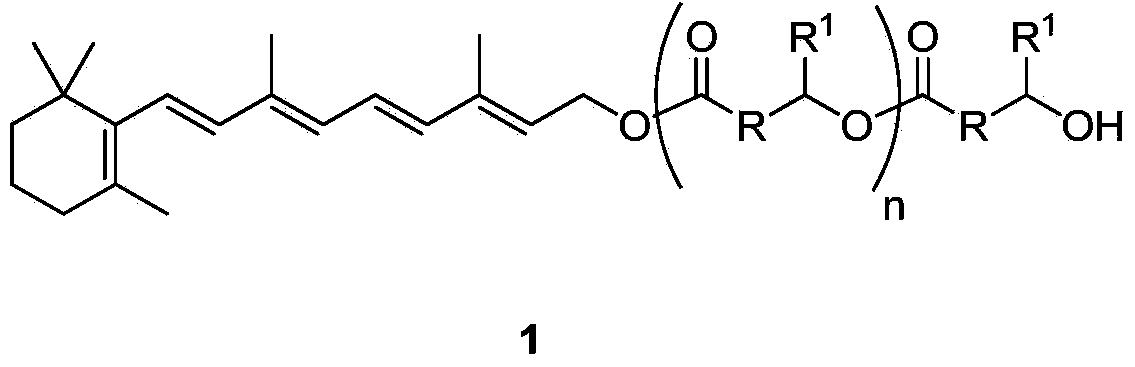
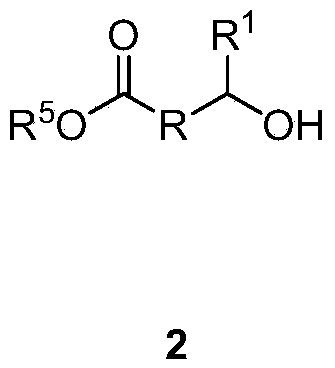
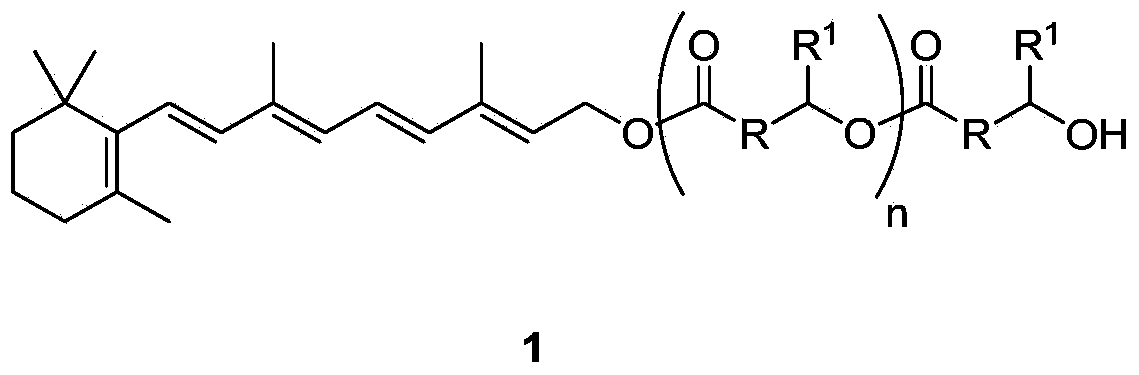
Hydroxyalkanoic acid and hydroxyalkanoic acid oligomer esters of retinol
A retinyl ester, hydroxyl technology, applied in the field of retinyl ester and its preparation, can solve the problems of increasing cost, time and waste, unstable retinol or retinyl ester degradation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of oligomers of retinyl-3-hydroxybutyrate (1a) (n=0-4)
[0064] In heptane, add retinol (58% retinol 25.9g, 15.0g retinol; 52.4mmol), ethyl 3-hydroxybutyrate (20.76g; 157mmol; 3 times equivalent) to the vial successively, and Novozym 435 (1.5g). The mixture was purged and stirred for 48 h at room temperature to obtain a mixture of oligomers of retinyl-3-hydroxybutyrate with a retinol conversion rate of 96.7%. The mixture was diluted with toluene (30ml), filtered to give a solid which was washed with toluene (30ml). The toluene solution was washed with water:methanol at a ratio of 1:1 (60ml), poured out the aqueous phase and extracted back with heptane (25ml), combined the organic layers, washed with water:methanol at a ratio of 1:1 (60ml), and washed with anhydrous sodium sulfate Dry and concentrate to give dark yellow oil 17.79g1a (R=CH 2 , R 1 =CH 3 ). HPLC (high performance liquid chromatography) analysis yielded 3.7% retinol and 95.5% 1a oligomers...
Embodiment 2
[0067] Preparation of retinyl ricinoleate (1b)
[0068] In toluene, add retinol (54% retinol 1.852g, 1.0g; retinol, 3.49mmol), ricinoleic acid (80% content; 1.250g; 4.19mmol; 1.2 times the equivalent ), and Novozym435 (1g). The mixture was sealed and stirred at room temperature for 21 h, and the conversion rate of retinol was 83% to obtain 1b.
[0069] HPLC (4.6x150mm Zorbax SB-C8 chromatographic column [Agilent], 3.5μ thickness, 90:10 methanol: water (containing 0.1% trifluoroacetic acid) detection time 7min, the gradient is converted into 95:5 methanol: water (containing 0.1% trifluoroacetic acid) within 1min 0.1% trifluoroacetic acid), maintain the concentration detection time for 12min, change the gradient to 100% methanol within 1min, maintain 100% methanol, and detect the wavelength at 325nm):t R 3.9min (retinol); t R 14.2min (1b).
Embodiment 3
[0071] Enzymatic hydrolysis of oligomers of retinyl-3-hydroxybutyrate (1a) (n=0-4)
[0072] The oligomer of retinyl-3-hydroxybutyrate (1a; 100 mg) was dissolved in 2 ml of toluene, a buffer solution (2 ml) with a pH value of 7 was added, Novozym 435 (100 mg) was added, and the mixture was vigorously stirred at room temperature , At 1, 24, and 48h, the supernatant was extracted and analyzed by HPLC. The results are shown in Table 1, and showed a large amount of hydrolysis at 48h. A control test without enzyme showed no hydrolysis after 48 hours.
[0073] Table 1 Enzymatic hydrolysis of oligomers of retinyl-3-hydroxybutyrate
[0074] time (h)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com