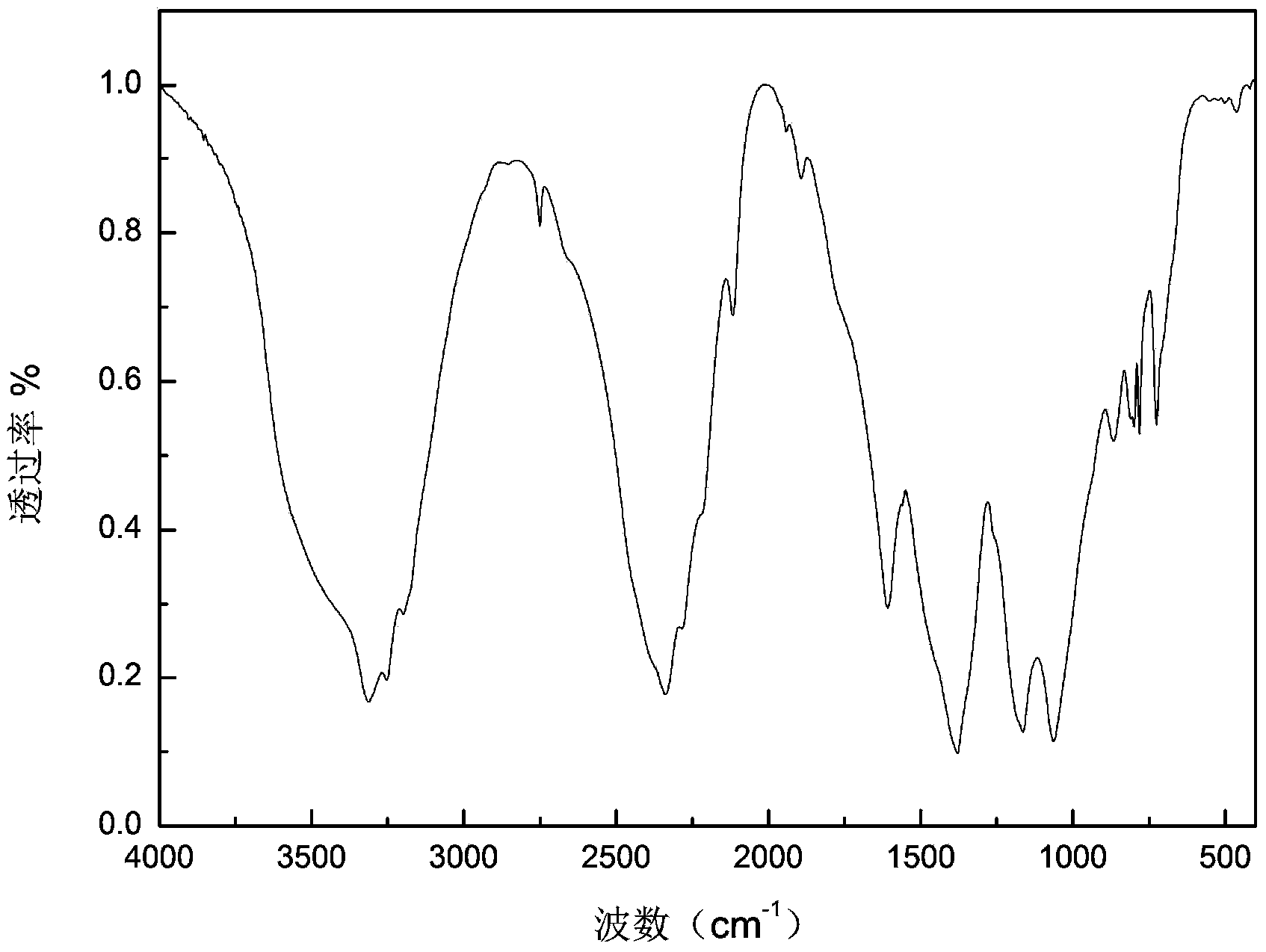
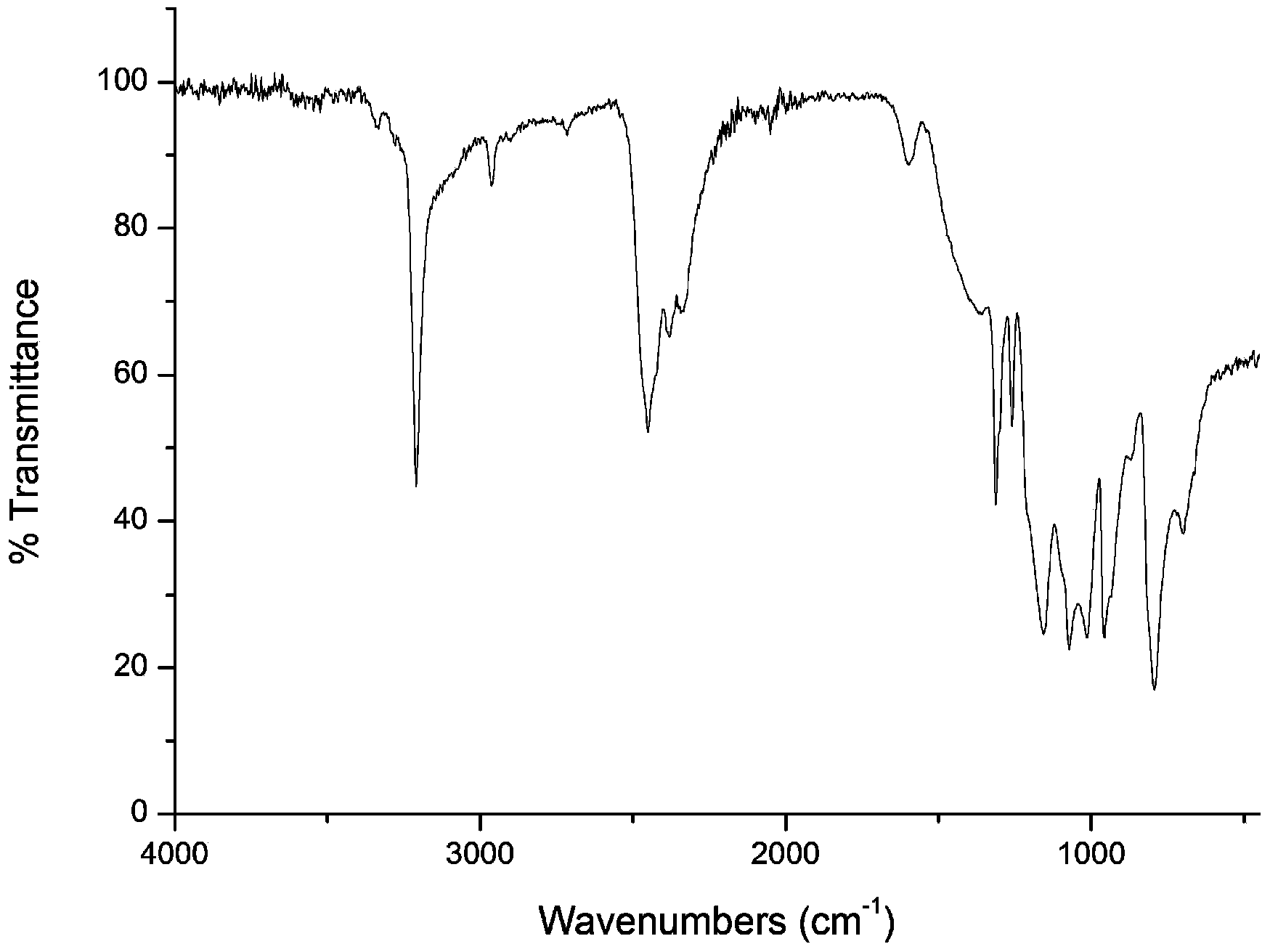
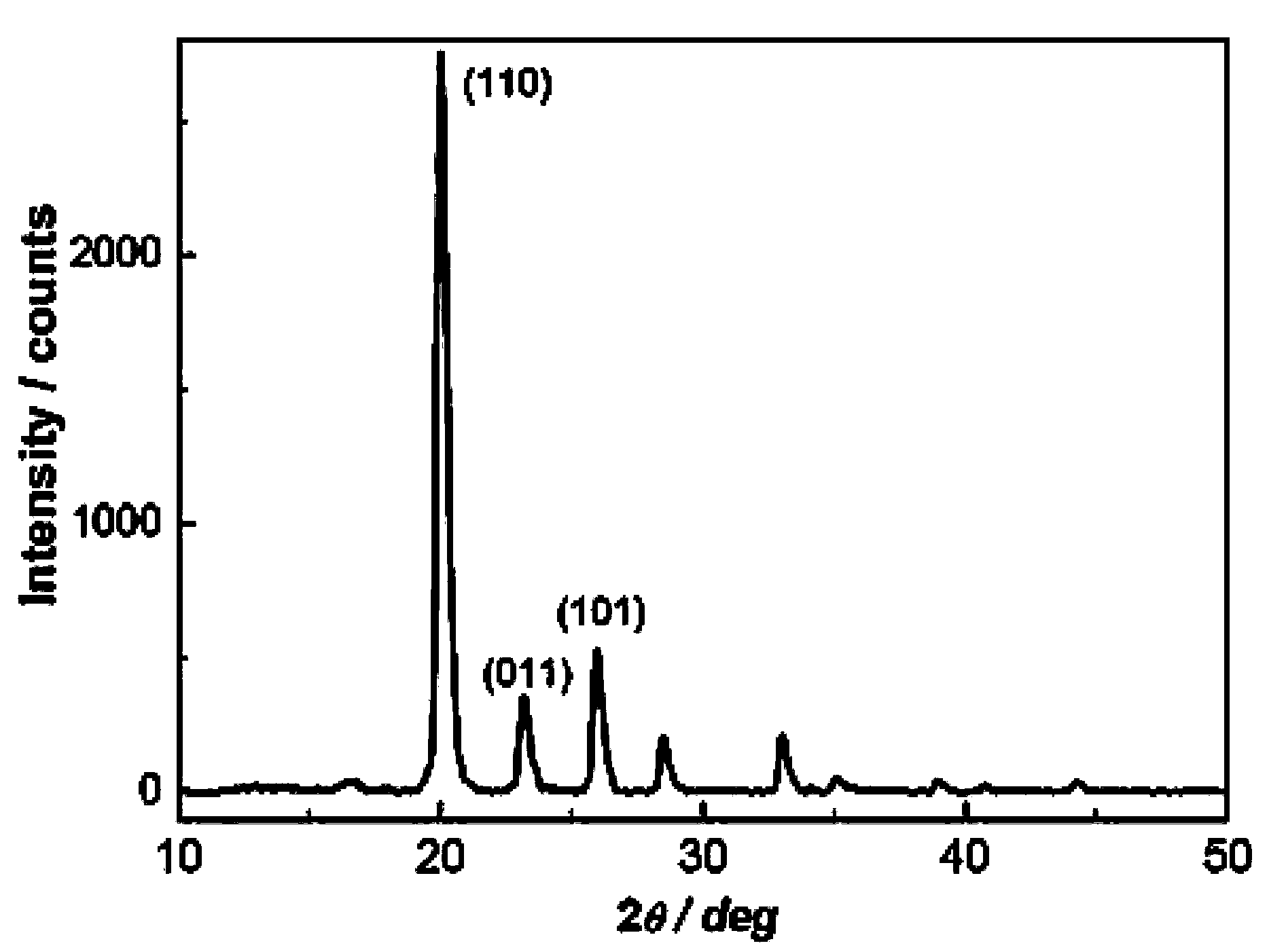
Preparation method of boron hydrogen nitrogen energy storage material
A technology of energy storage materials and hydrogen storage materials, which is applied in the production of hydrogen, etc., can solve the problems of boron hydrogen nitrogen material raw materials, low product purity, high processing cost, etc., and achieve high yield, high purity, and reduced pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 1.01mol of (NH 4 ) 2 SO 4 Put it into a 5L three-necked reaction flask, which is equipped with a thermometer, a polytetrafluoroethylene stirring paddle, and a spherical reflux device. The top of the reflux pipe is connected with a moisture-proof tube. anhydrous solvent, stir for 30min;
[0027] (2) Add 2mol NaBH 4 Add to the ammonium salt solution in step (1), stir for 30 minutes, then heat up to 40°C, and react for 5 hours;
[0028] (3) The mixed solution in step (2) was filtered, and the filtrate was subjected to rotary evaporation to obtain 58 g of white solid powder, with a yield of 94%;
[0029] (4) Dissolve the white powder obtained in step (3) in tetrahydrofuran anhydrous solvent to form a saturated solution, add the saturated solution dropwise to petroleum ether solvent, when microcrystals appear, under the condition of -5°C After standing for 24 hours, a white crystalline product was obtained, and the crystallization was repeated 3 times; the cryst...
Embodiment 2
[0033] (1) Add 0.55mol of (NH 4 ) 2 CO 3 Add to the three-necked reaction flask filled with 2L 2-methylfuran anhydrous solvent, and install a spherical reflux condenser, thermometer and Teflon stirring paddle on the three-necked reaction flask, and stir for 30 minutes;
[0034] (2) Add 1mol NaBH 4 Add it to the ammonium salt solution in step (1), stir for 30 minutes, then raise the temperature to 42°C, and react for 4 hours;
[0035] (3) Filter the mixed solution in step (2), and perform rotary evaporation on the filtrate to obtain 29.32 g of white powder, with a yield of 95%;
[0036] (3) Dissolve the white solid powder in tetrahydrofuran anhydrous solvent to form a saturated solution, sublime in an oven at 60°C and a vacuum of 0.02 for 20h to obtain BNH 6 White crystals 21.91g, BNH 6 The purity reaches 99.4%.
Embodiment 3
[0038] (1) Add 0.26mol of (NH 4 ) 2 CO 3 Add it to a 1L four-necked reaction flask, which is equipped with a polytetrafluoroethylene stirring device, a thermometer, a feeding tube, and a spherical reflux device, and the top of the reflux tube is connected with a moisture-proof tube. Anhydrous solvent, stirring for 20min;
[0039] (2) Add 0.5mol LiBH 4 Add to the ammonium salt solution in step (1), stir for 30 minutes, heat up to 43°C, and react for 3.5 hours;
[0040] (3) The mixed solution of (2) was filtered, and the filtrate was subjected to rotary evaporation to obtain 14.5 g of white solid powder, with a yield of 94%;
[0041] (4) Dissolve the white solid powder obtained in step (3) in tetrahydrofuran anhydrous solvent to make a saturated solution, and sublimate the saturated solution in an oven with a temperature of 60°C and a vacuum of 0.02 for 12 hours to obtain white BNH 6 Crystalline product 12.6g, BNH 6 The purity reaches 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com