Catalyst for dehydrogenation of light alkane and preparation method of catalyst
A technology of low-carbon alkanes and catalysts, which is applied in the field of catalysts and preparations for the dehydrogenation of low-carbon alkanes, and can solve the problems of catalyst stability and selectivity are not very good, performance is not very ideal, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
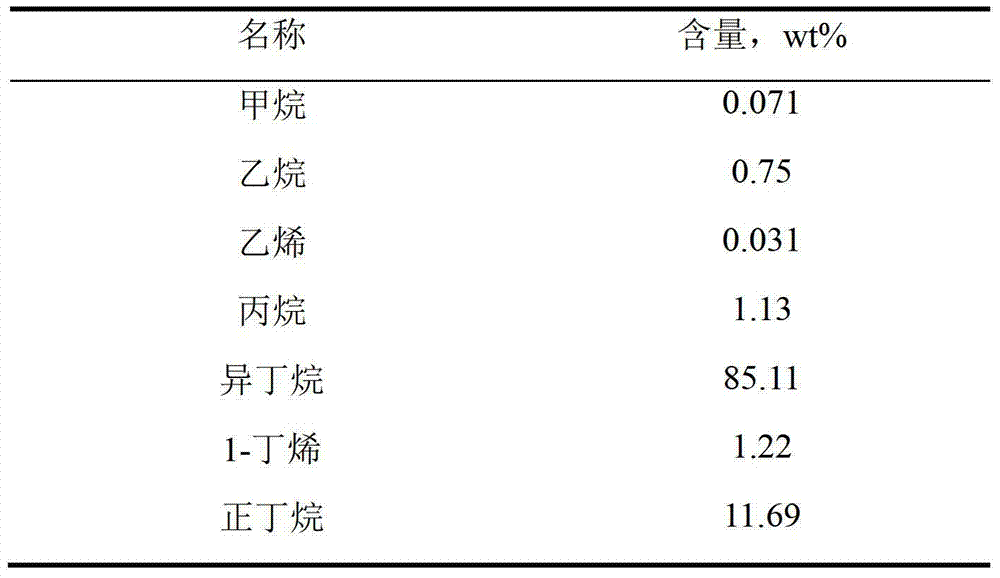
[0018] In the laboratory, a fixed-bed reactor is used with an inner diameter of 20mm and a length of 50cm. The reactor is wrapped with an open electric heating furnace, which can continuously supply the heat required for the reaction. The raw material composition is shown in Table 1, which contains 85.11% (weight) of isobutane and 12.69% (weight) of n-butane. The reaction temperature is 600°C, and the space velocity of low-carbon alkanes is 1000h -1 , the volume ratio of hydrogen and raw materials is 0.5:1, the two are mixed into the top of the reactor after metering, the reactor is equipped with a catalyst, the catalyst is a self-made noble metal catalyst with platinum as the active component, and the catalyst contains platinum 0.35 %, tin 0.5%, take a sample after reacting for 1 hour, analyze the product composition with a gas chromatograph, remove the hydrogen content, and the product composition is shown in Table 2.
[0019] Table 1
[0020]
[0021] Table 2
[002...
Embodiment 2
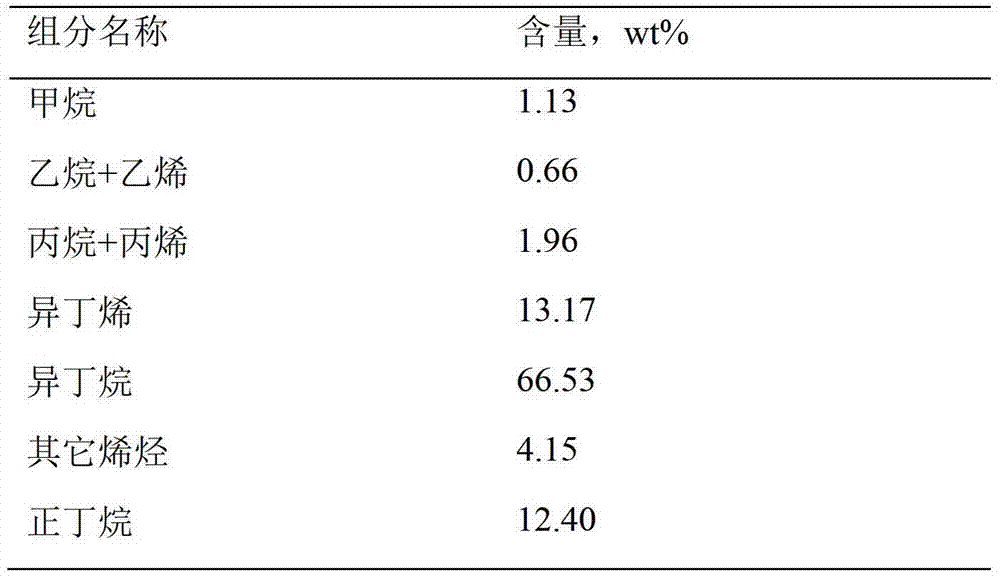
[0030] Reactor and reaction conditions as embodiment 1, reaction raw material is isobutane, purity 99.94%, adopt Pt-Sn catalyzer and Pt-Sn-Ce-La-K catalyzer, contain platinum 0.35% in the catalyzer, tin 0.7%, K0.2% (weight), Ce0.25% (weight), La0.27% (weight), the composition of the reaction product is shown in Table 5.
[0031] table 5
[0032]
[0033] It can be seen from Table 5 that for high-purity isobutane raw materials, the selectivity and conversion rate of the catalyst are greatly improved after the Pt-Sn-Ce-La-K catalyst is used. By changing the ratio of La and K, it is found that under the condition that K accounts for 0.1-0.5% (weight) of the carrier and La accounts for 0.01-1.0% (weight) of the carrier, the selectivity and conversion rate of the catalyst can be effectively improved.
Embodiment 3
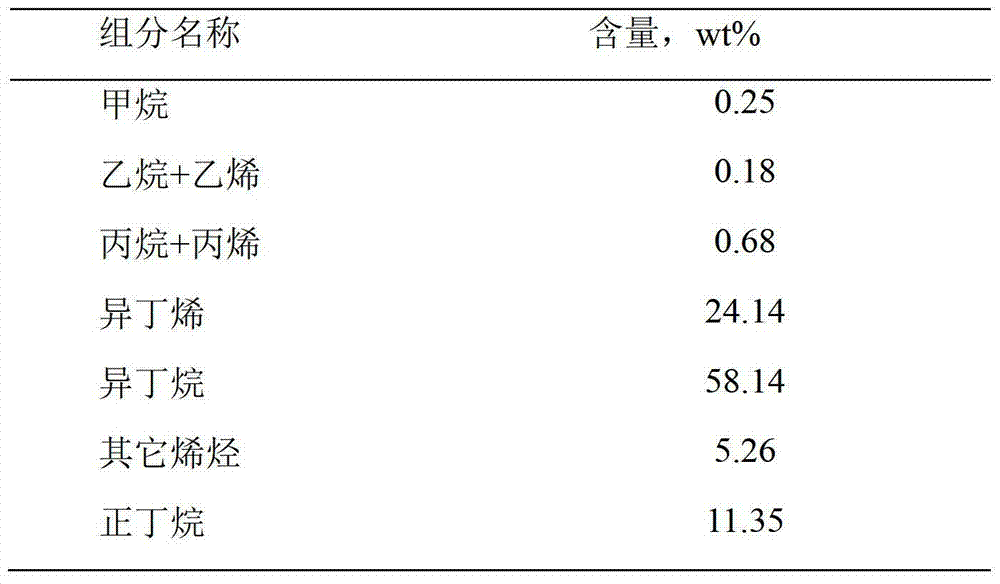
[0035] Using the same reactor and catalyst as in Example 1, the reaction temperature is controlled at 610 ° C, and other conditions are the same as in Example 2, using propane feed, propane purity 99.9%, propane normal pressure space velocity is 1000h -1 , The volume ratio of propane to hydrogen is 1:0.5, and the results are shown in Table 6 after 4 hours of reaction.
[0036] Table 6
[0037]
[0038] It can be seen from Table 6 that the dehydrogenation effect of propane using Pt-Sn-Ce-La-K catalyst is better than that of Pt-Sn catalyst currently in use in the world, and the conversion rate and selectivity are higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com