Environment-friendly process unit and process for continuous stable production of electrolytic nickel or electrolytic cobalt
A technology for stabilizing production and process equipment, which is applied in the field of environmentally friendly electrolytic nickel or electrolytic cobalt continuous and stable production process equipment to achieve the effects of saving electricity, stable product quality, and stable pH value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
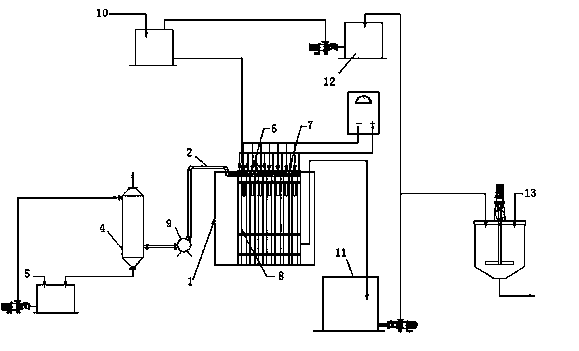
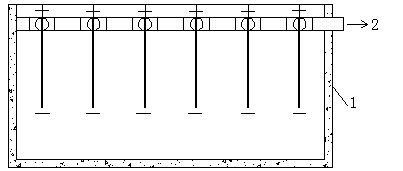
[0037] The device for electrolytic nickel or electrolytic cobalt in the present invention comprises an electrowinning cell, a cathode frame, an anode frame, a circulation system and an acid mist recovery system, the cathode frame and the anode frame are all arranged in the electrowinning cell, and the cathode frame and the anode frame A permeable membrane is set between them, the cathode and anode frames are all airtight structures, all the cathode frames and anode frames are connected together by screws, the electrolyte inlet is set on the cathode frame, and the acid mist absorption port is set on the anode frame, the acid mist absorption The acid mist absorption port is connected to the acid mist absorption pipe, and is connected to the acid mist recovery system through the acid mist absorption port. The anolyte outlet is set at the lower end of the anode frame, and each anolyte to the outlet is connected to the manifold, and is connected to the circulation system through the ...
Embodiment 2
[0043] In the configured electrolyte composition, the content of nickel is 80g / L, cobalt 0.004 g / L, copper 0.0005 g / L, zinc 0.0005g / L, manganese 0.0005 g / L, iron 0.0005g / L, cadmium 0.0005g / L, lead 0.003 g / L, Mg 8.0, Ca 0.24g / L, Cr 0.001g / L, Na 25g / L, organic matter 0.001g / L, H 2 BO 3 11.0g / L, Cl - 0.07g / L, pH=3 (measured by pH test paper); electrolysis conditions: current density 400A / m 2 , electrolyte temperature 60°C, electrolyte circulation 40m 3 / t.Ni, cell voltage 4.0v, DC power consumption 5000 Kw.h, current efficiency99%, cathode and anode liquid level difference is 20mm.
[0044] A cathode frame and an anode frame are set in the electrowinning cell, and a 3751 permeable membrane is set between the cathode frame and the anode frame. The electrolyte in the high-level tank enters the electrowinning cell through the liquid supply main pipe, and then passes through the electrolyte inlet set on the cathode frame. , the catholyte enters the cathode frame, and then enters...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com