Devilstongue gulcomannan hemostatic sponge and preparation method thereof
A technology of konjac glucomannan and hemostatic sponge, which is applied in medical science, absorbent pads, bandages, etc., can solve the problems of uneven pore size, rough konjac sponge, poor color and luster, and achieve uniform pore size, air permeability and water absorption rate. High and flexible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
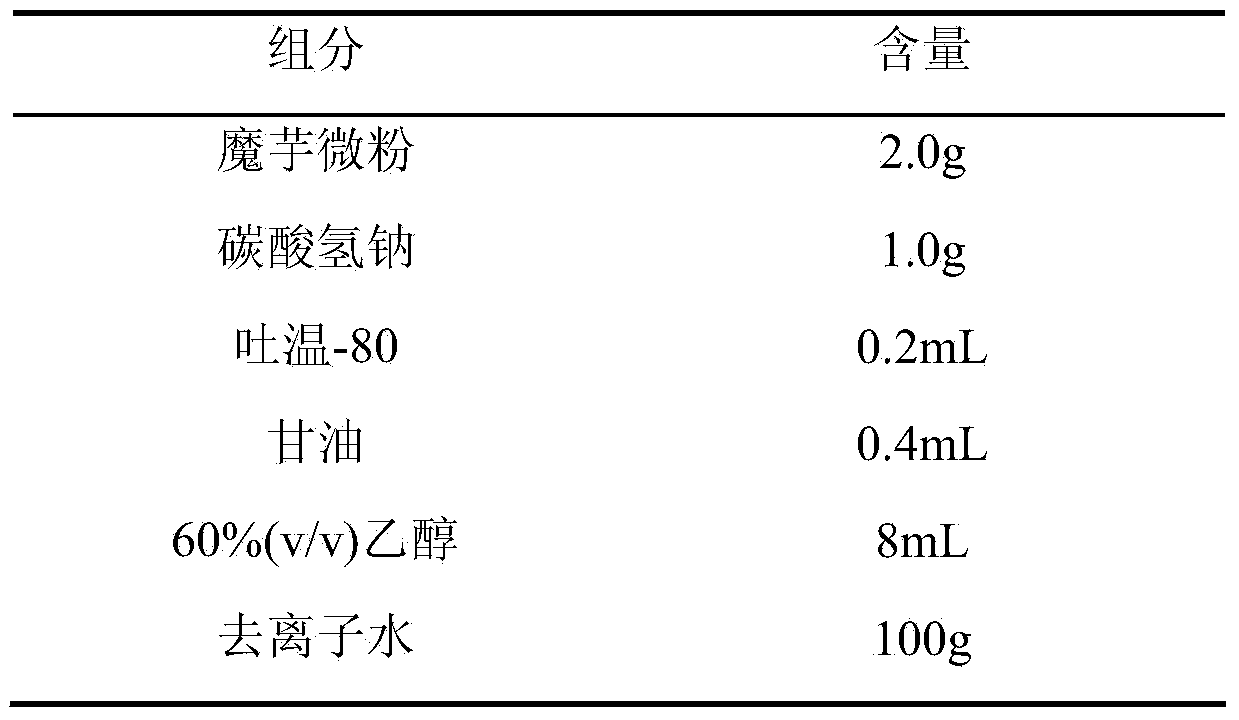
[0014] formula:
[0015]
[0016] Preparation process: Weigh konjac powder, add it to deionized water, heat at 40°C, dissolve and expand for 30 minutes, stir until the colloid is smooth and fine, then add sodium bicarbonate to dissolve, add Tween 80 and glycerin while it is hot, and stir to produce uniform Fine foam, then add 60% ethanol to solidify, transfer the resulting solution to a mold, control the thickness of 1mm, pre-freeze at -20°C for 24 hours, then vacuum dry for 24 hours, cut, pack, Co 60 Sterilize by irradiation to obtain konjac glucomannan hemostatic sponge.
Embodiment 2
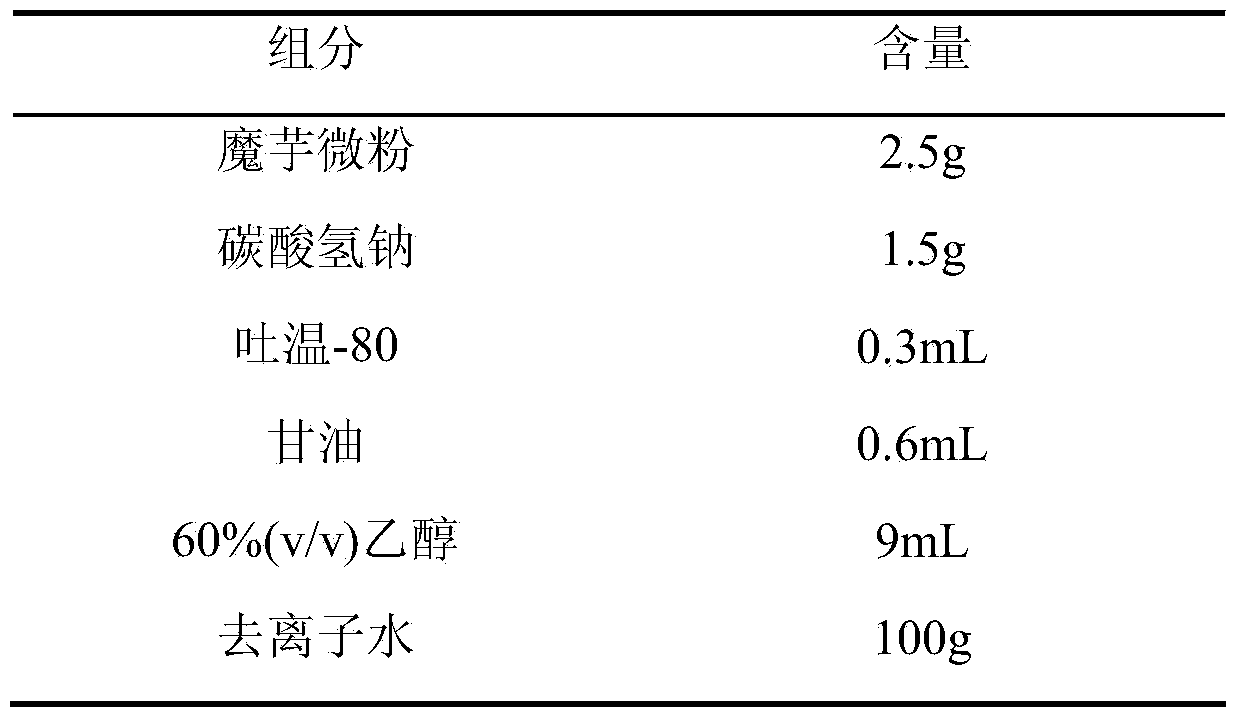
[0018] formula:
[0019]
[0020] Preparation process: Weigh konjac powder, add it to deionized water, heat at 45°C, dissolve and expand for 35 minutes, stir until the colloid is smooth and fine, then add sodium bicarbonate to dissolve, add Tween 80 and glycerin while it is hot, and stir to produce uniform Fine foam, then add 60% ethanol to solidify, transfer the resulting solution to a mold, control the thickness of 2mm, pre-freeze at -20°C for 24 hours, then vacuum dry for 30 hours, cut, pack, Co 60 Sterilize by irradiation to obtain konjac glucomannan hemostatic sponge.
Embodiment 3
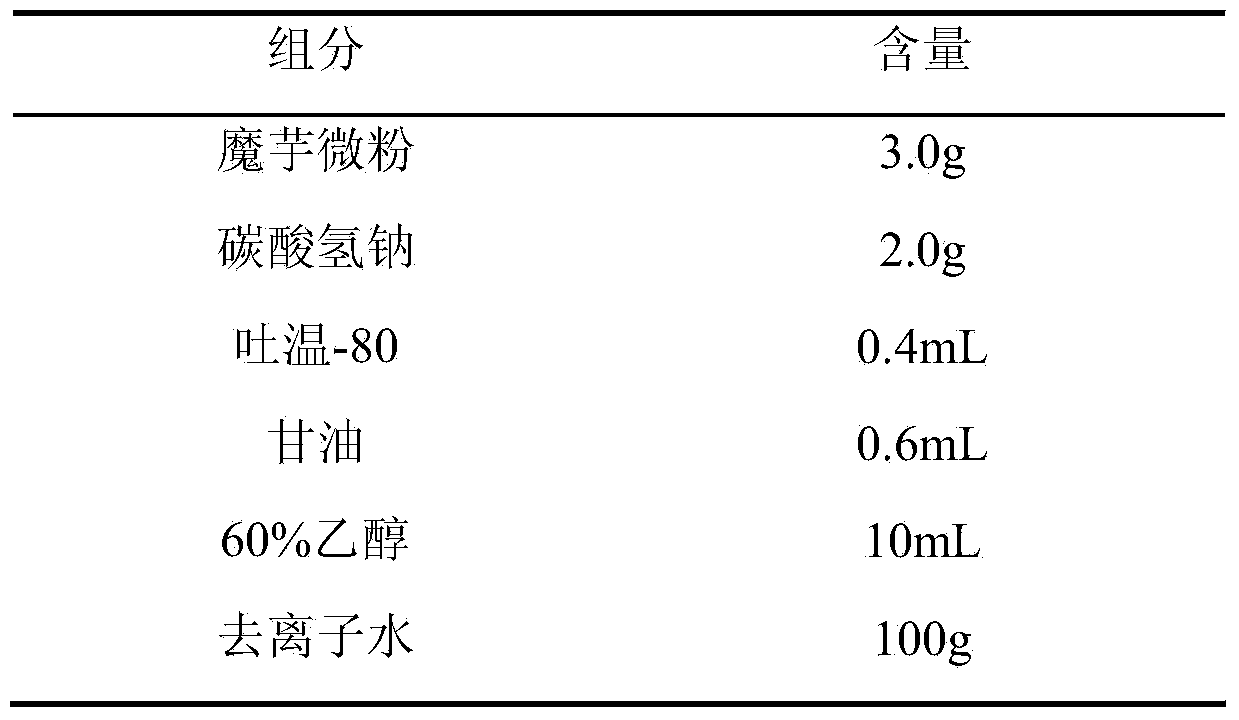
[0022] formula:
[0023]
[0024] Preparation process: Weigh konjac powder, add it to deionized water, heat at 50°C, dissolve and expand for 40 minutes, stir until the colloid is smooth and fine, then add sodium bicarbonate to dissolve, add Tween 80 and glycerin while it is hot, and stir to produce uniform Fine foam, then add 60% ethanol to solidify, transfer the resulting solution to a mold, control the thickness of 3mm, pre-freeze at -20°C for 24 hours, then vacuum dry for 36 hours, cut, pack, Co 60 Sterilize by irradiation to obtain konjac glucomannan hemostatic sponge.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com