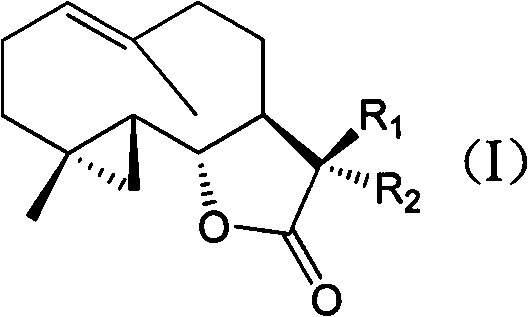
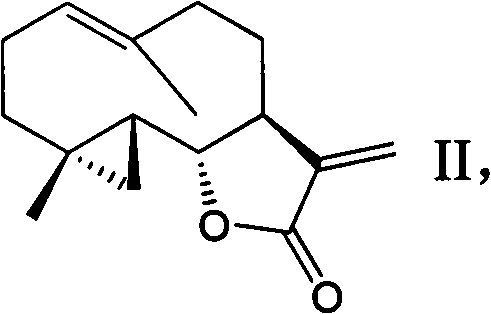
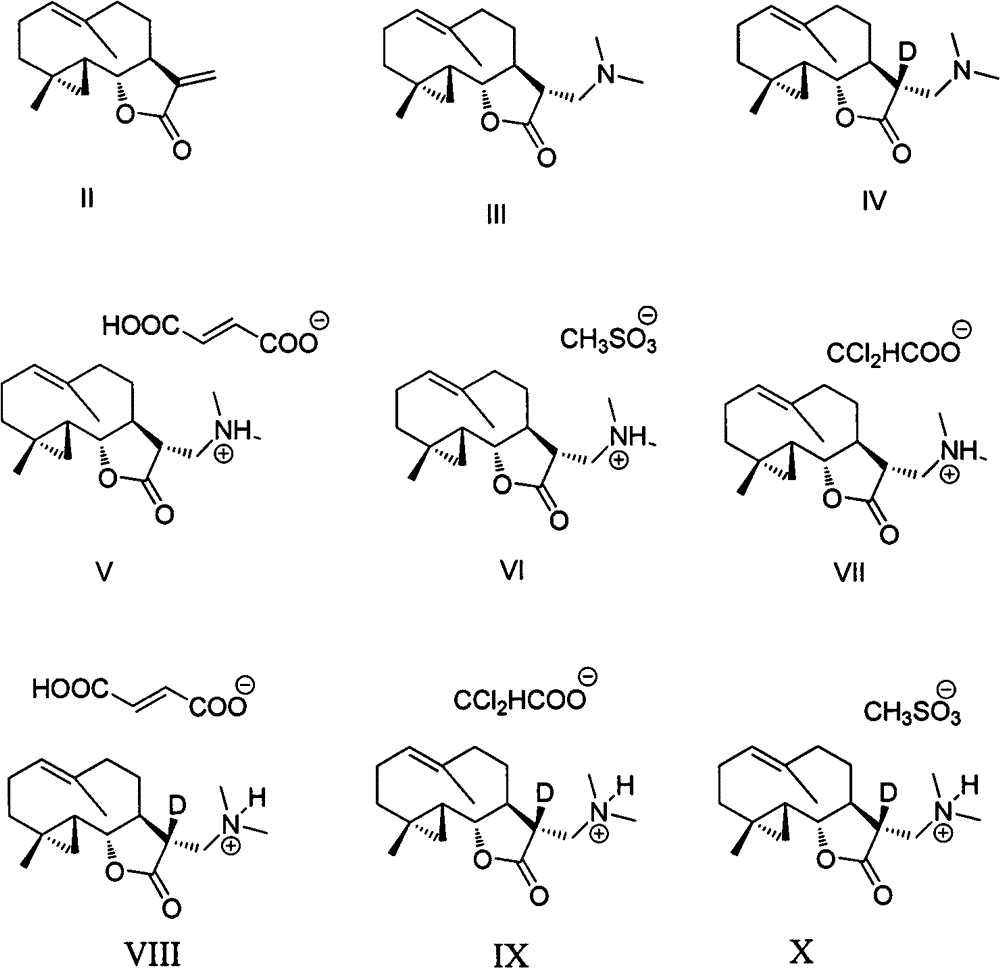
Costunolide derivative and pharmaceutical composition, preparation method and uses thereof
A technology of woody lactone and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Structural formula is as follows the preparation route of formula II:
[0032]
[0033] Preparation of compound 1:
[0034] Under the protection of nitrogen, dissolving cosmolide (2.0g, 8.6mmol) in dried dichloromethane, cooled to zero, slowly added 1M diisobutylaluminum hydride dichloromethane solution (10.6mL, 10.6mmol ), continue stirring for two hours after adding, after TLC detects that the reaction is substantially complete, quench with saturated ammonium chloride solution (100mL), filter a layer of diatomaceous earth, extract the aqueous phase three times with dichloromethane, and combine the dichloromethane layers , washed with saturated brine, and dried over anhydrous sodium sulfate. After filtration and purification by concentration column chromatography, 1.37 g (68%) of the hemiacetal intermediate was obtained. Dissolve 1.27g of hemiacetal in 40mL of methanol, cool to zero, add sodium borohydride (427mg, 11.3mmol) in batches, stir for four hours, TLC sho...
Embodiment 2
[0048] The synthetic method of compound III:
[0049]
[0050] Take compound II (590mg, 2.4mmol), dimethylamine hydrochloride (2.94g, 36mmol) was dissolved in 120mL of dichloromethane, 9.95g of potassium carbonate was added, refluxed for 5 hours, filtered, spin-dried, and obtained by silica gel column chromatography Compound III (645 mg).
[0051] 1 H NMR (400MHz, CDCl 3 )δ 5.17 (br d, J = 10.2Hz, 1H), 3.76 (t, J = 7.9Hz, 1H), 2.71 (dd, J = 13.0, 5.2Hz, 1H), 2.55 (dd, J = 13.0, 5.2Hz, 1H), 2.46-2.36(m, 1H), 2.36-2.29(m, 1H), 2.25(m, 8H), 2.17-2.10(m, 1H), 2.07-1.95(m, 3H), 1.77 -1.69(m, 1H), 1.68(s, 3H), 0.98(s, 3H), 0.88-0.71(m, 2H), 0.53-0.49(m, 1H), 0.44(t, J=5.4Hz, 1H ); 13 C NMR (100MHz, CDCl 3 )δ177.6, 133.4, 127.7, 85.0, 58.6, 51.3, 47.9, 46.2, 41.2, 38.8, 32.4, 31.9, 25.4, 20.2, 18.2, 18.0, 16.7.
Embodiment 3
[0053] The synthetic method of compound IV:
[0054]
[0055] Take compound II (167 mg), deuterated dimethylamine hydrochloride (530 mg) was dissolved in 15 mL of dichloromethane, 1.8 g of potassium carbonate was added, stirred for 24 hours, filtered, spin-dried, and passed through the column to obtain compound IV (150 mg, 76% ).
[0056] 1 H NMR (400MHz, CDCl 3 )δ5.15(br d, J=11.4Hz, 1H), 3.74(t, J=7.9Hz, 1H), 2.69(d, J=12.8Hz, 1H), 2.53(d, J=12.8Hz, 1H ), 2.45-2.33(m, 1H), 2.32-2.17(m, 8H), 2.16-2.09(m, 1H), 2.06-1.93(m, 3H), 1.78-1.62(m, 4H), 0.97(s , 3H), 0.83-0.73(m, 2H), 0.50(dd, J=9.3, 4.9Hz, 1H), 0.43(t, J=5.3Hz, 1H).; 13 CNMR (100MHz, CDCl 3 )δ176.6, 132.3, 126.7, 84.0, 57.5, 50.2, 46.9, 45.2, 40.2, 37.8, 31.3, 30.9, 24.4, 19.2, 17.2, 17.0, 15.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com