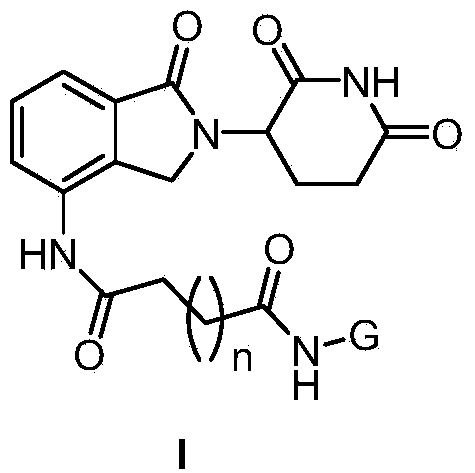
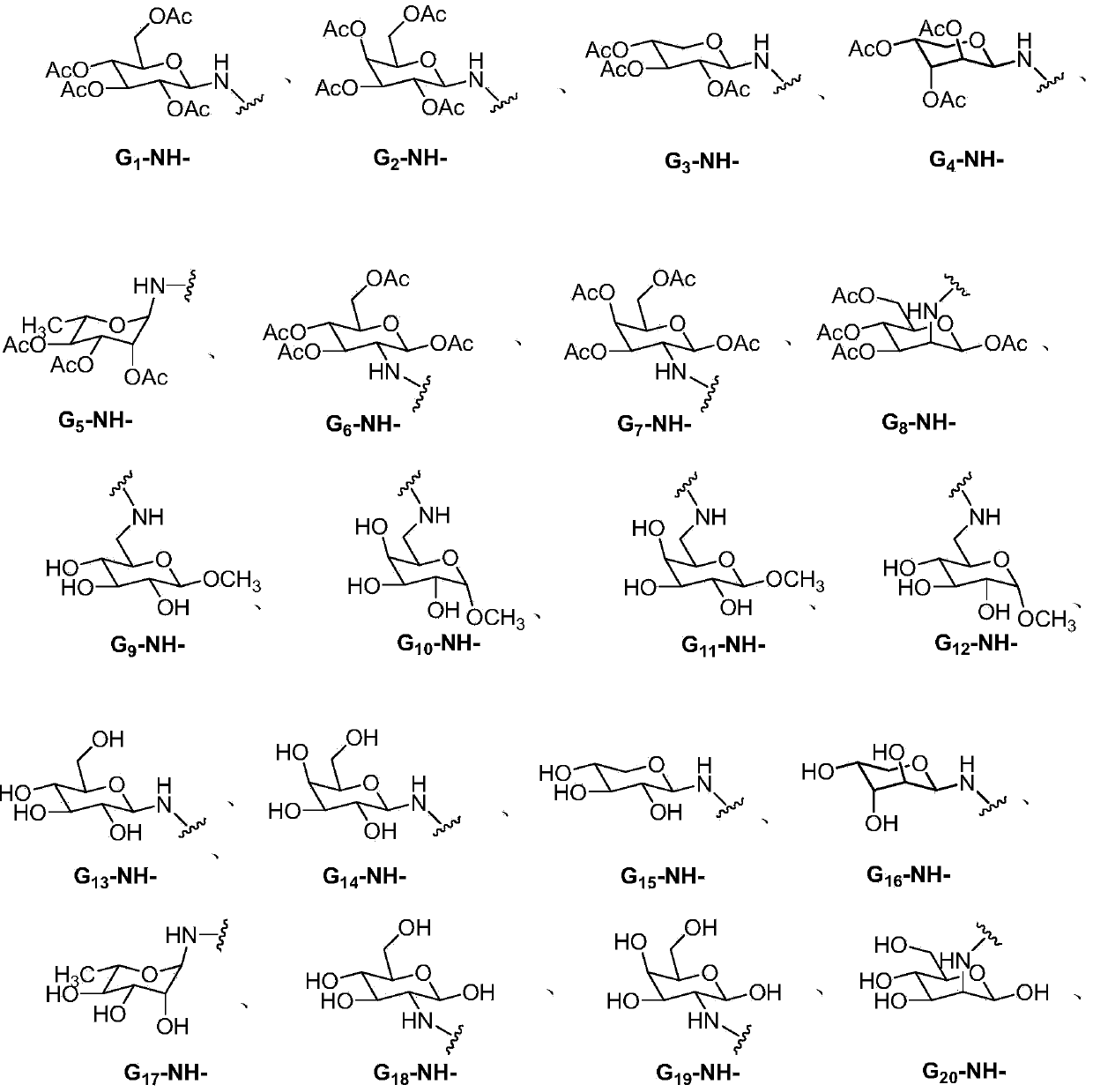
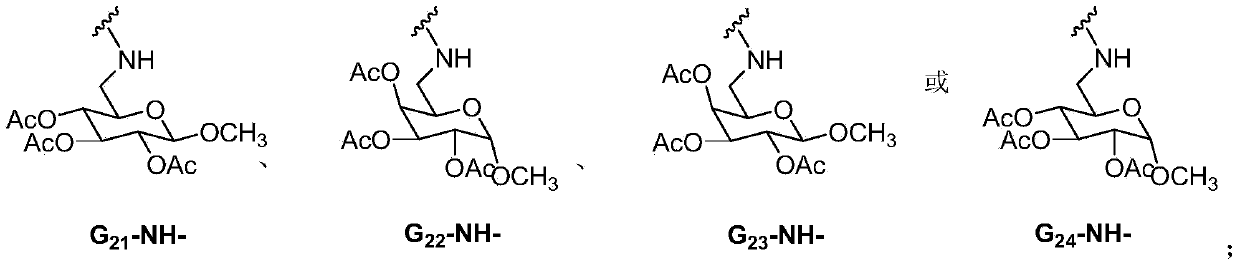
Lenalidomide derivative and preparation method and pharmaceutical application thereof
A drug and pharmaceutical technology, applied in the direction of preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve problems such as long-term use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157]N-(2,3,4,6-tetra-O-acetyl-1-deoxy-β-D-glucopyranosyl)-4-(2-(2,6-dioxopiperidine-3- Preparation of -1-oxoisoindoline-4-amino)-4-oxobutyramide 1.5 hydrate (I-2)
[0158] N-Benzyloxycarbonyl-L-glutamine (1)
[0159] Dissolve L-glutamine (58.4g, 0.4mol) in K 2 CO 3 (82.8g, 0.6mol) in water (400ml). Benzyl chloroformate (85ml, 0.5mol) was added dropwise in an ice-water bath, and after the addition was complete, the mixture was stirred at room temperature for 3h. The reaction solution was extracted with ethyl acetate (300ml×3), the aqueous layer was acidified with concentrated HCl until the pH value was 2-3 (no more white turbidity), and placed in the refrigerator overnight for crystallization. Suction filtration, grind the filter cake with a mortar, wash the obtained white solid with water, and dry under an infrared lamp to obtain 82.0 g of a white solid, with a yield of 73.1%, m.p.132~133 (literature value: m.p.133~135°C [Justus Liebigs Annalen der Chemie,1961,640,145-1...
Embodiment 2
[0201] N-(2,3,4,6-tetra-O-acetyl-2-deoxy-β-D-glucopyranosyl)-5-(2-(2,6-dioxopiperidine-3- Preparation of -1-oxoisoindoline-4-amino)-5-oxopentamide dihydrate (I-3)
[0202] 5-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline-4-amino)-5-oxopentanoic acid (7-3)
[0203] Lenalidomide (3.93g, 15mmol) was dissolved in DMF (30ml), glutaric anhydride (2.58g, 22.5mmol) was added, heated to 60°C for 48h. Pour the reaction solution into ether, stir to precipitate a large amount of solids, place in the refrigerator overnight to continue crystallization, and filter. After washing with ether, 5.15 g of light yellow solid was obtained, with a yield of 93.0%, m.p.150-152°C;
[0204] 1 H-NMR (300MHz, DMSO-d 6 )δ (ppm): 12.08 (1H, brs, COOH), 11.03 (1H, s, CONHCO), 9.82 (1H, s, CONH), 7.82 (1H, s, aromatic), 7.50 (2H, s, aromatic) ,5.15(1H,d,J=11.7Hz,NCHCO),4.34(2H,s,PhCH 2 ),2.92~2.87(2H,m,CONHCH 2 CH 2 C H 2 COOH), 2.63~2.53 (2H, mCONHC H 2 CH 2 CH 2 COOH),2.40(2H,s,CH 2 C H 2 CO...
Embodiment 3
[0216] N-(2,3,4,6-tetra-O-acetyl-1-deoxy-β-D-galactopyranosyl)-4-(2-(2,6-dioxopiperidine-3 Preparation of -yl)-1-oxoisoindoline-4-amino)-4-oxobutyramide (I-5)
[0217] 1,2,3,4,6-penta-O-acetyl-β-D-galactopyranose (8-2)
[0218] Anhydrous NaOAc (2.5 g, 30.4 mmol) was suspended in Ac 2O (35ml), heated to reflux. Control the reflux speed, add D-galactopyranose (5.0 g, 0.029 mol) in batches, and continue to reflux for 1 hour. After cooling, the reaction solution was poured into crushed ice (about 250 ml), stirred for 4 hours, and a solid was precipitated. Filter and wash with cold water to obtain 6.5 g of off-white solid, recrystallize from absolute ethanol (50 ml) to obtain 5 g of white crystal, yield 44.2%. m.p.147-149°C. (Literature value mp.144-146°C [CN1594342A])
[0219] 1-bromo-2,3,4,6-tetra-O-acetyl-1-deoxy-α-D-galactopyranose (9-2)
[0220] In a three-necked flask, suspend red phosphorus (0.7g, 5.6mmol) in acetic acid (7.7ml), stir well, and slowly add liquid bromi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com