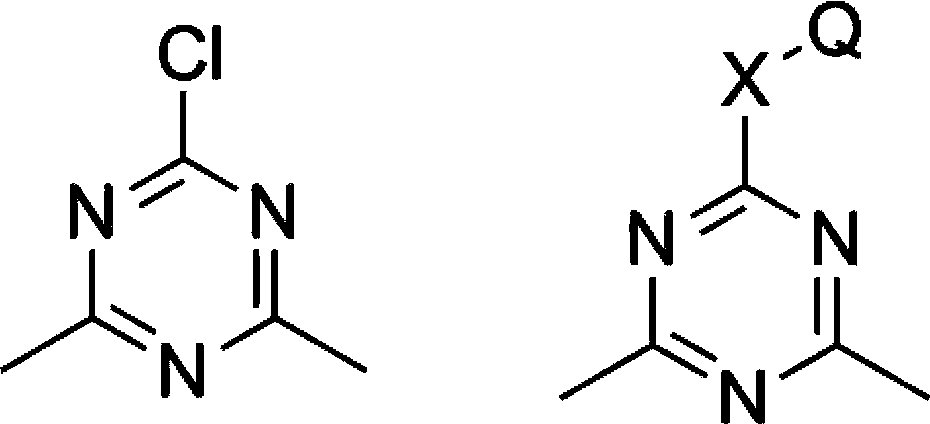
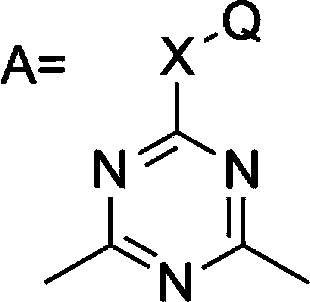
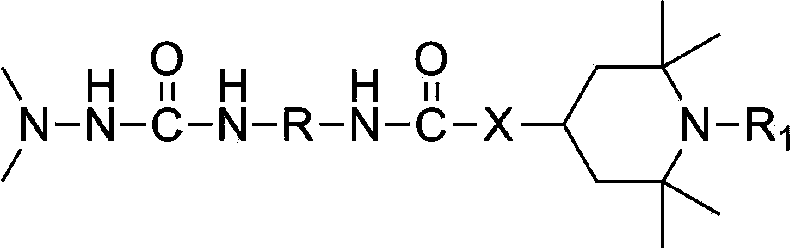L1,1-dimethylhydarzine stabilizerand the stabilizer composition thereof
A technology of dimethyl hydrazine and stabilizer, applied in the field of dimethyl hydrazine-type stabilizer, can solve the problems such as the inability to improve the light resistance of organic polymer materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment one: synthetic compound A
[0096] In a 500 ml four-necked mechanical stirring bottle, 250 ml of toluene and 40 g (0.238 mol) of 1,6-hexyl diisocyanate were added after replacing with nitrogen, and 14.3 g (0.2383 mol) of 1,1- 37.3 grams (0.238 moles) of dimethylhydrazine will be exothermic during the dripping process, and the temperature will naturally rise to 50-55 ° C. After one hour of reaction, add 37.3 grams of tetramethylpiperidinol and maintain the temperature at 55-60 ° C. After two hours of reaction, the temperature was raised to 112° C. to evaporate the toluene and the residual toluene was evaporated by a water-circulating vacuum motor to obtain 89 grams of white solid. Compound structures and results are shown in Table (1).
Embodiment 2
[0097] Embodiment two: synthetic compound B
[0098] In a 500 ml four-necked mechanical stirring bottle, add 250 ml of toluene and 50 g (0.2 moles) of 4,4-methylene bis(phenylisocyanate) after replacing with nitrogen, and slowly add 31.2 gram (0.2 mol) of tetramethylpiperidine ammonia, there will be exothermic phenomena during the dripping process, and the temperature will naturally rise to 40-45°C, and 12.6 gram (0.21 mol) of 1,1-dimethylhydrazine will be added after reacting for one hour During the dropping process, solids will be produced. Maintain the temperature at 55-60°C for three hours, then raise the temperature to 90°C, react for two hours and then cool down to 10°C to filter out the crystals and obtain 85 grams of light yellow solid. Compound structures and results are shown in Table (1).
Embodiment 3
[0099] Embodiment three: synthetic compound C
[0100] In a 500 ml four-necked mechanical stirring bottle, add 250 ml of toluene and 47 g (0.188 moles) of 4,4-methylene bis(phenylisocyanate) after replacing with nitrogen, and slowly add 32.1 g of (0.188 moles) pentamethylpiperidinol and 0.1 gram of stannous octoate (T9), there will be exothermic phenomena during the addition process, and the temperature will naturally rise to 40-45 ° C. After two hours of reaction, add 11.5 grams (0.191 moles) 1 , 1-Dimethylhydrazine, maintain the temperature at 55-60°C for three hours, then raise the temperature to 90°C, after two hours of reaction, heat up to 112°C to evaporate toluene and use a circulating water vacuum motor to evaporate the residual toluene to obtain 86 grams white solid. Compound structures and results are shown in Table (1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com