Method for separating umbilical cord mesenchymal stem cell effectively
A technology of mesenchymal stem cells and umbilical cords, which is applied in the field of separation and preparation of human umbilical cord mesenchymal stem cells, can solve the problems of isolated cell contamination, inability to completely remove bacteria and other microorganisms, and achieve the goal of less cell damage, less pollution, and lower costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Isolation, culture and cryopreservation of human umbilical cord mesenchymal stem cells
[0024] (1) Separation of umbilical cord mesenchymal stem cells: The umbilical cord tissue ligated at both ends was washed with sterile isotonic solution, and the umbilical cord ligated at both ends can basically ensure that the inner wall of the umbilical vein is not polluted. Cut off 1-2 cm inside the ligation at one end, insert a sterile tube of appropriate length and diameter from the umbilical vein at the cut end, and leave a tube of more than 2 cm outside the umbilical cord (the length and diameter of the tube can be adjusted according to the umbilical vein, Make sure that it can be firmly inserted into the umbilical cord vein, and leave a tube of more than 2cm outside the umbilical cord), and fix the umbilical cord and tube at about 1-2 cm inside the umbilical cord end (for the method of intubation, see figure 1 ), inject 10-25 ml of sterile isotonic solution from th...
Embodiment 2
[0027] Example 2 Expansion and Preparation of Human Umbilical Cord Mesenchymal Stem Cells
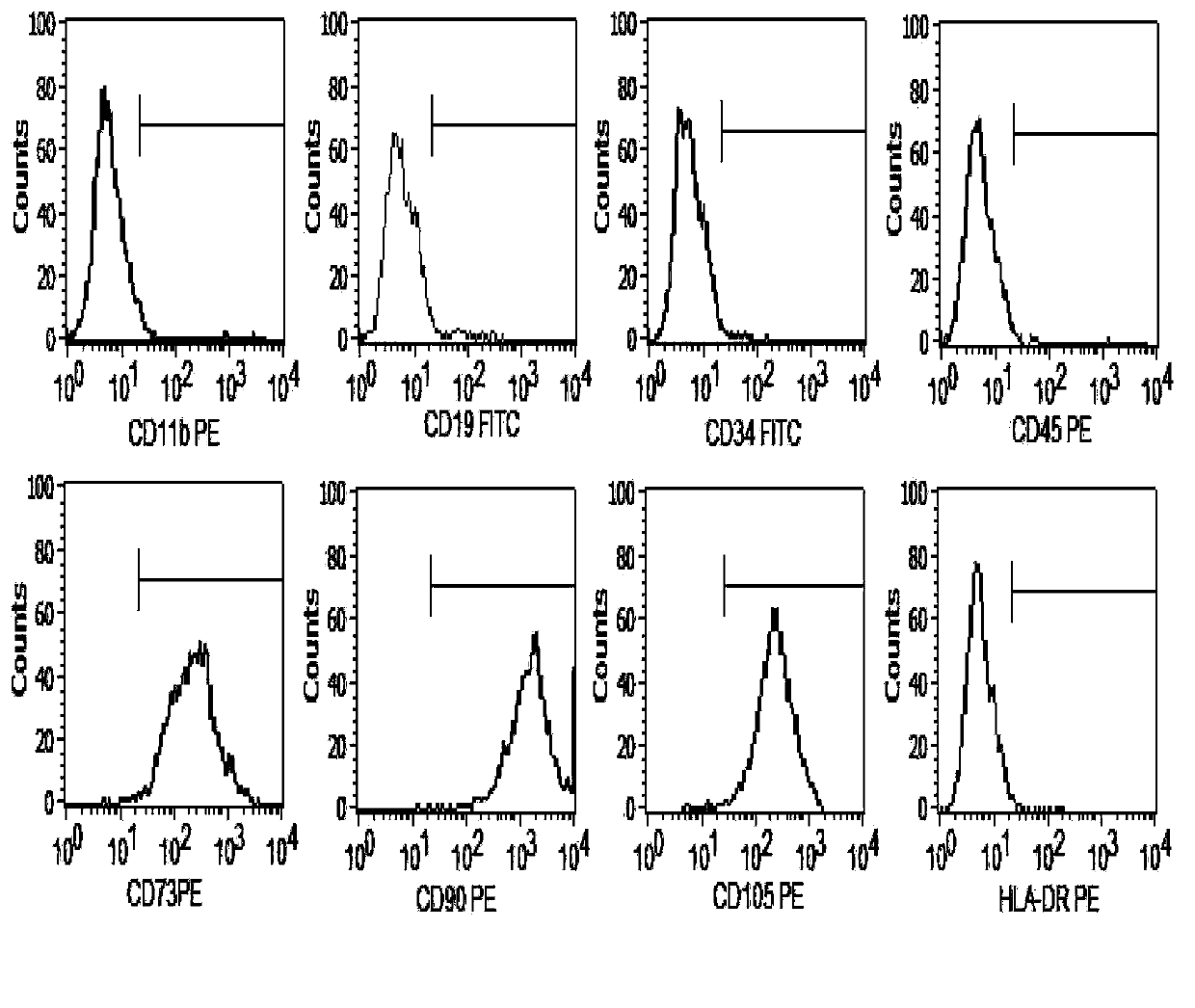
[0028] (1) Take 3×10 cells from qualified primary passage cell bank 6 Cell cryopreservation tubes, thaw and recover cells at 37°C, (2) press 2×10 with serum-free medium (LONZA) 4 / cm 2 The density was inoculated into culture flasks, and cultured in a 37°C, saturated humidity, 5% CO2 incubator. When the cells reached 80-90% confluence, they were passaged at a ratio of 1:3. After 3 passages, (3) Use trypLE when the confluency of the third passage reaches 90% TM Digest the cells, count them, resuspend the cells with the prepared cell protection solution at an appropriate concentration, divide them into sterile containers, seal them, and store them in a suitable environment. (4) Take part of the preparation and use the methods known to professionals to conduct pathogenic microorganism inspection of bacteria, fungi and viruses, cell purity inspection, cell viability detection, endotoxin d...
Embodiment 3
[0030] Example 3 Effect of separation method on contamination of umbilical cord mesenchymal stem cells obtained by natural delivery
[0031] The method of this patent and the method of mechanically crushing the umbilical cord and digesting them with collagenase and trypsin were used to separate 80 normal umbilical cords. The sterility test was performed on the umbilical cord collection fluid and the primary culture fluid supernatant cultured after isolation, and the success rate of cell rescue was compared. The calculation formula is as follows:
[0032] Cell rescue success rate = (1-primary supernatant contamination / umbilical cord collection fluid contamination) × 100% Result: Using this patented method: 17 cases of umbilical cord collection fluid contamination, no primary supernatant contamination after separation, and cell rescue The success rate was 100%. However, the method of mechanically crushing the umbilical cord and digesting it with collagenase trypsin: 15 cases of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com