Preparation method of drug bearing microsphere/chitosan/sodium alginate injectable aquogel
A technology of drug-loaded microspheres and sodium alginate, which is applied in the field of preparation of biomedical materials, can solve the problems of poor drug release performance and low mechanical strength, and achieve the effects of enhanced mechanical strength, easy availability of raw materials, and weakened burst release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Dissolve 0.35 g chitosan in 2% 10 ml dilute acetic acid aqueous solution to obtain solution 1; dissolve 0.3 g sodium alginate in 10 ml 80 ℃ deionized water to obtain solution 2; dissolve 0.03 g Tris Dissolve in 1 ml of deionized water to obtain solution 3; dissolve 0.75 g of anhydrous calcium chloride in 3 ml of deionized water to obtain solution 4;
[0023] (2) Stir the solutions 1, 2, and 3 obtained in step (1) at room temperature at 470 rpm to obtain a mixed solution; add 0.105 g of drug-loaded microspheres of 300-450 um prepared in advance to the mixed solution, and use The quality machine was dispersed evenly at a high speed of 7000 rpm to obtain a suspension, which was stored in a freezer at 4 °C for 2 h;
[0024] (3) Inject the suspension into the defect site, and after 7 min, after initial coagulation, inject 2.1 ml of solution 4; the drug-loaded hydrogel is obtained.
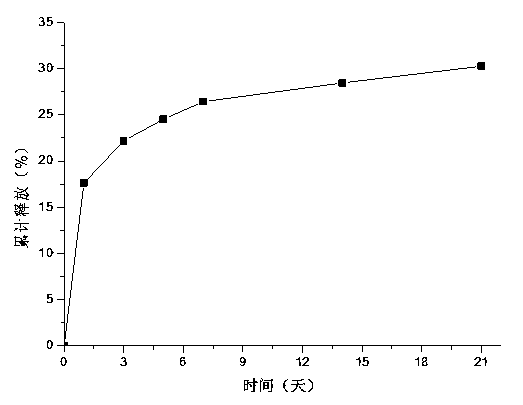
[0025] In vitro drug release curve experiment of composite hydrogel: add 2 mg drug-loade...
Embodiment 2
[0027] (1) Dissolve 0.1 g chitosan in 1% 10 ml dilute acetic acid aqueous solution to obtain solution 1; dissolve 0.12 g sodium alginate in 2 ml 70 ℃ deionized water to obtain solution 2; dissolve 0.05 g Tris Dissolve in 10 ml of deionized water to obtain solution 3; dissolve 1.5 g of anhydrous calcium chloride in 2 ml of deionized water to obtain solution 4;
[0028] (2) Stir the solutions 1, 2, and 3 obtained in step (1) at room temperature at 300 rpm to obtain a mixed solution; add 0.88 g of drug-loaded microspheres of 200-300 um prepared in advance to the mixed solution, and use the homogenizer The quality machine was 2000 rpm at high speed to disperse evenly to obtain a suspension, which was stored in a freezer at 0 °C for 4.5 h;
[0029] (3) Inject the suspension into the defect site, and after 3 min, after initial solidification, inject 1.47 ml of solution 4; the drug-loaded hydrogel is obtained.
[0030]The drug-loaded microspheres / chitosan / sodium alginate injectable ...
Embodiment 3
[0032] (1) Dissolve 0.18 g chitosan in 3% 10 ml dilute acetic acid aqueous solution to obtain solution 1; dissolve 0.15 g sodium alginate in 15 ml 60 ℃ deionized water to obtain solution 2; dissolve 0.75 g Tris Dissolve in 5 ml deionized water to obtain solution 3; dissolve 3 g anhydrous calcium chloride in 6 ml deionized water to obtain solution 4;
[0033] (2) Stir the solutions 1, 2, and 3 obtained in step (1) at room temperature at 600 rpm to obtain a mixed solution; add 0.6 g of drug-loaded microspheres of 100-200 um prepared in advance to the mixed solution, and use the homogenizer The quality machine was dispersed evenly at a high speed of 4700 rpm to obtain a suspension, which was frozen at 8 °C for 8 h;
[0034] (3) Inject the suspension into the defect site, and after 10 min, after initial coagulation, inject 6 ml of solution 4; the drug-loaded hydrogel is obtained.
[0035] The drug-loaded microspheres / chitosan / sodium alginate injectable hydrogel prepared in this e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com