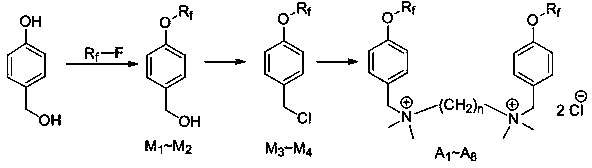
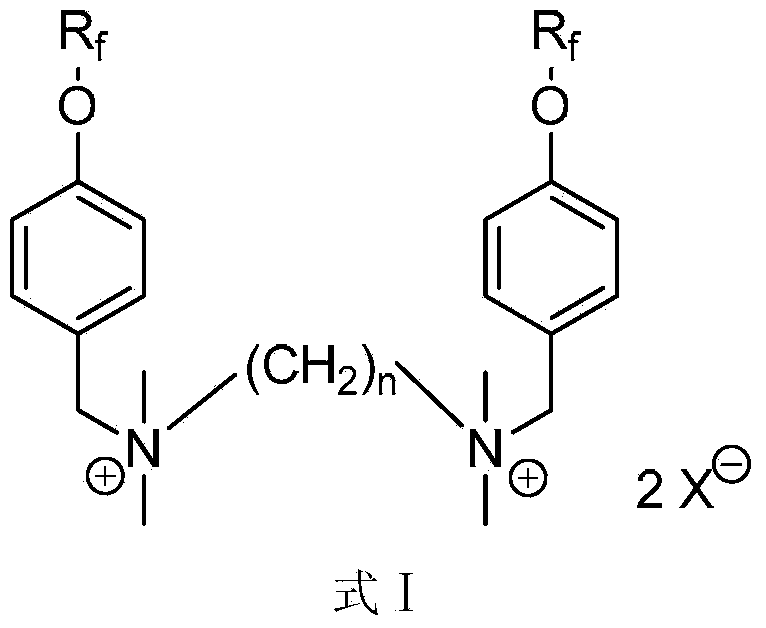
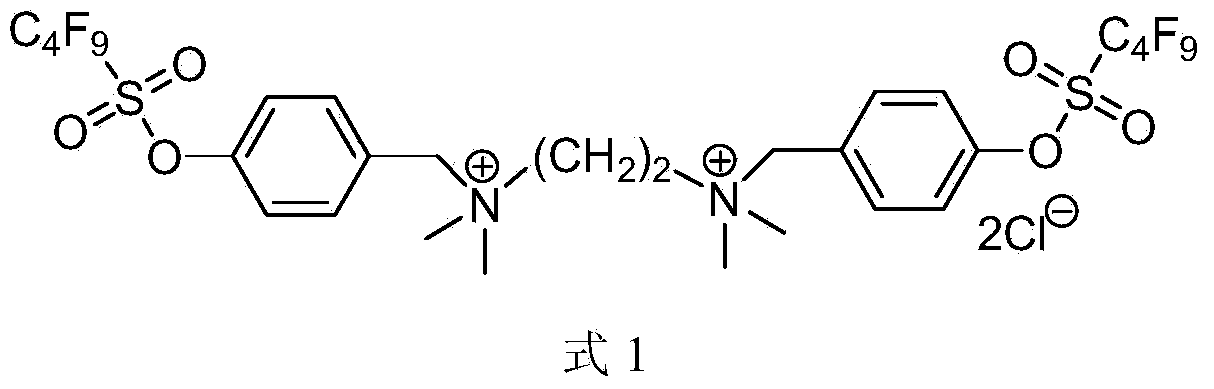
Gemini perfluoroalkylsulfonyloxybenzyl cationic surfactant as well as preparation and application thereof
A technology of perfluoroalkylsulfonyloxybenzylcation and perfluorobutylsulfonyloxy, which is applied in the field of surfactants, can solve the problems of high environmental hazards and unsatisfactory fire extinguishing effect, and achieve fire extinguishing performance Excellent, water-forming film-forming effect with fast spreading speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 intermediate (M 1 , M 2 ) preparation
[0032] Add 12.4g (0.1mol) p-hydroxybenzyl alcohol, 17.9g (0.13mol) potassium carbonate, 200mL acetonitrile into a dry 1000mL flask, heat to reflux, slowly add 52.26g (0.13mol) perfluorobutylsulfonyl fluoride dropwise, The reaction was continued for 2.5 h, and the end point of the reaction was monitored by TCL. Add 150mL ethyl acetate to the reaction solution, wash 3 times with saturated sodium chloride solution, the organic layer is dried over anhydrous sodium sulfate, and solvent removal is obtained to obtain the colorless transparent liquid intermediate perfluorobutanesulfonic acid-4-(hydroxy Methyl) phenyl ester (M 1 ), 35.7g, the yield is 88%. 1 H NMR (400MHz, CDCl 3 ):δ2.50(s,1H,OH),4.61(s,2H,CH 2 ),6.89(d,2H,J=7.8Hz,phH),7.33(d,2H,J=7.8Hz,phH); 19 F NMR (376MHz, CDCl 3 ):δ-128.827(2F),-123.802(2F),-111.125(2F),-84.248(3F); MS(EI):406(M + ) (calculated value: 405.99).
[0033] Add 12.4g (0.1mol) p-hydro...
Embodiment 2
[0034] Embodiment 2 intermediate (M 3 , M 4 ) preparation
[0035] Add 44.66g (0.11mol) perfluorobutanesulfonic acid-4-(hydroxymethyl)phenyl ester (M 1 ), 200mL of 1,4-dioxane, slowly add 38.6g (0.22mol) of thionyl chloride dropwise under ice bath, react for 30min after the drop, raise the temperature to 60°C and continue the reaction for 1h, and monitor the end point of the reaction by TLC. Slowly poured into ice water, added 200mL ethyl acetate, washed three times with saturated sodium chloride solution, dried the organic layer over anhydrous sodium sulfate, and precipitated to obtain a colorless liquid intermediate perfluorobutanesulfonic acid-4-(chloro Methyl) phenyl ester (M 3 ), 37.7g, the yield is 81%. 1 H NMR (400MHz, CDCl 3 ):δ4.56(s,2H,CH 2 ),6.90(d,2H,J=3.9Hz,phH),7.40(d,2H,J=4.2Hz,phH); 19 F NMR (376MHz, CDCl 3 ):δ-128.827(2F),-123.802(2F),-111.125(2F),-84.248(3F); MS(EI):424(M + ) (calculated value: 423.95).
[0036] Add 55.6g (0.11mol) perfluorohexylsul...
Embodiment 3
[0037] Example 3N, N, N'N'-tetramethyl-N, N'-bis((4-perfluorobutylsulfonyloxy)benzyl)ethane-1,2-diammonium salt (A 1 )Synthesis
[0038] Add 6.36g (0.015mol) perfluorobutanesulfonic acid-4-(chloromethyl)phenyl ester (M 3 ), 0.8g (7mmol) tetramethylethylenediamine, 150mL anhydrous acetonitrile, reflux reaction overnight, suction filtration, the obtained solid was washed three times with ether, and finally a white solid was obtained as the target compound (A 1 ), 6.41g, the yield is 95%. 1 H NMR(600MHz,DMSO):δ3.18(s,12H,N(CH 3 ) 2 ),4.096(s,4H,CH 2 ),4.762(s,4H,CH 2 ),7.71(d,4H,J=7.8Hz,phH),7.85(d,4H,J=12.0Hz,phH); 19 F NMR (376MHz, DMSO): δ-128.827 (2F), -123.802 (2F), -111.125 (2F), -84.248 (3F); HPLC / MS (ESI): 931.1 (M + ) (calculated value: 930.09).
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com