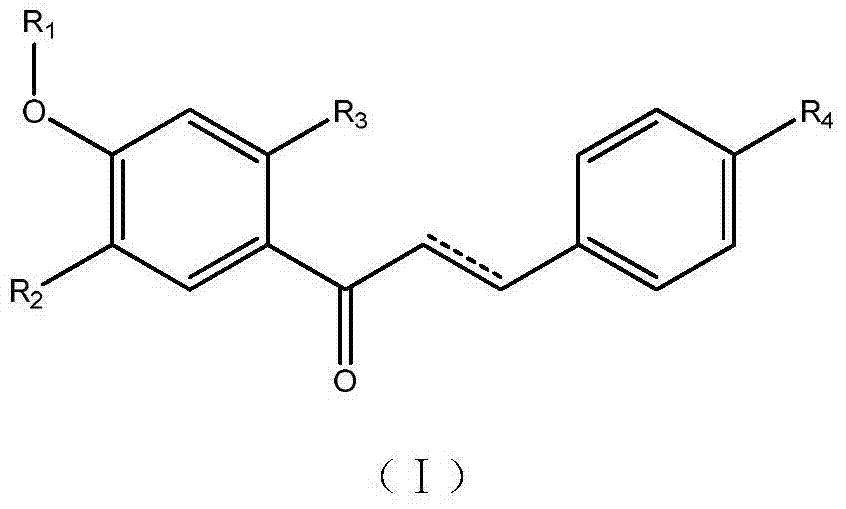
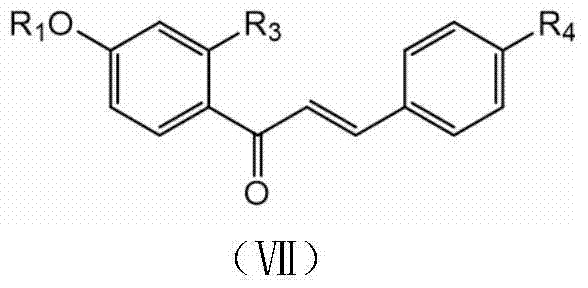
Compound with antibacterial synergism as well as preparation method and application thereof
A compound and chemical formula technology, applied in the field of compounds with antibacterial synergistic effect and their preparation, can solve problems such as inability to cure, can not catch up with the speed of generation of drug-resistant bacteria, etc., to achieve the effect of improving antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1: the synthesis of p-dimethylaminobenzaldehyde
[0115] In a 100ml three-necked flask, add 4.9g (0.032mol) of phosphorus oxychloride, and slowly add 2.5g (0.031mol) of DMF dropwise under ice bath. 3.66g (0.03mol) N,N-dimethylaniline (10min), after dropping, move the reaction solution to a boiling water bath for 2 hours, after the reaction, pour the reaction solution into 20ml of ice water, and oxidize it with 30% hydrogen The sodium solution was adjusted to PH=4, left to stand for crystallization, and suction filtered the next day to obtain a light yellow or nearly colorless solid, which was recrystallized with 30ml of ethanol-water (1:2.5V / V) to obtain the product p-dimethylaminobenzaldehyde .
Embodiment 2
[0116] Embodiment 2: the synthesis of 2,4-dihydroxyacetophenone
[0117] Add 11.00g (0.1mol) of resorcinol and 30ml of glacial acetic acid in sequence to a 100ml three-necked flask equipped with a condenser, agitator, and a thermometer, raise the temperature to 145°C, and react at a constant temperature for 5 hours. After the reaction is complete, pour the reaction solution into 50ml of dilute hydrochloric acid (1:9), static crystallization, suction filtration, and recrystallization with ethanol-water (1:9V / V). In 2,4-dihydroxyacetophenone.
Embodiment 3
[0118] Example 3: Synthesis of 4-dimethylamino-2, 4-dihydroxychalcone
[0119] in N 2 Under protection, add 1.52g (0.010mol) 2,4-dihydroxyacetophenone, 1.94g (0.013mol) p-dimethylaminobenzaldehyde, DMSO 3ml, piperidine 1ml, Heat the oil bath to 150°C and react for 10 minutes. After the reaction is complete, pour the reaction solution into 50ml of 10% sodium hydroxide solution, filter with suction, acidify the filtrate with concentrated hydrochloric acid to PH=1-2, stand for crystallization, and filter with suction. The obtained solid was recrystallized from 15 ml of ethanol to obtain 1.60 g of 4-dimethylamino-2□,4□-dihydroxychalcone as a yellow solid, with a yield of 56.5%. 1 HNMR (400MHz, Acetone-d 6):δ3.13(s,6H,4-CH 3 );6.42(d,1H,3′-H);6.52(dd,J=8.9Hz,1H,5′-H);6.85(d,J=8.9Hz,2H,3-H,5-H) ,7.73(d,J=15.2Hz,1H,a-H);7.77(d,J=8.9Hz,2H,2-H,6-H);7.91(d,J=15.2Hz,1H,b-H);8.21 (d,J=8.9Hz,1H,6′-H);9.45(s,1H,4′-OH);13.92(s,1H,2′-OH)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com