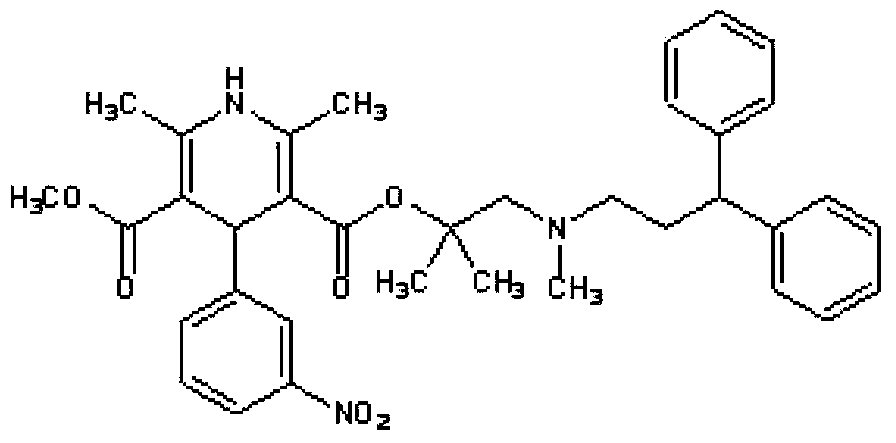
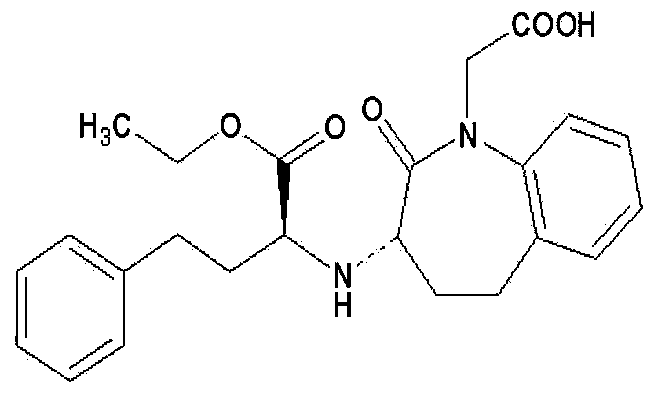
Solid medicine composition of lercanidipine and benazepril
A technology of lercanidipine and its composition, which is applied in the field of medicine, can solve problems affecting drug absorption speed and degree, affecting drug safety and effectiveness, and unsatisfactory dissolution rate, so as to reduce treatment costs, improve clinical application value, Good synergistic antihypertensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
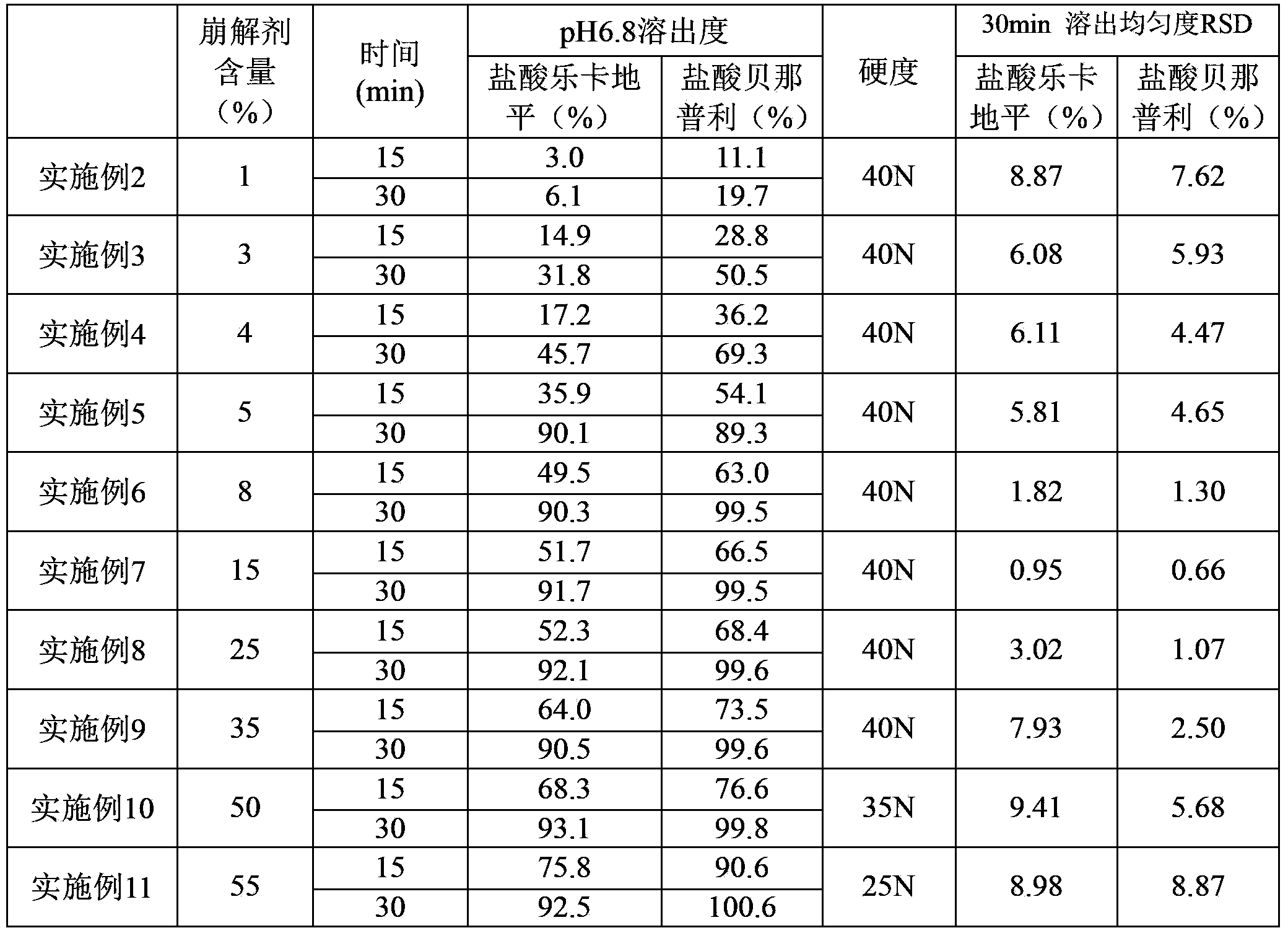
Image
Examples
Embodiment 1
[0050] Calculate and weigh lercanidipine hydrochloride, benazepril hydrochloride, appropriate amount of filler and disintegrant, put them into separate marked containers, transfer the above drugs and auxiliary materials to the stirring granulator in turn, and use a low speed Stir and shear mix for 5 minutes to obtain a mixture. The mixture was passed through a 40-mesh sieve, and the sieved mixture was again sheared and mixed in a stirring granulator.
[0051] Prepare the binder into a 70% ethanol-water (w / w) solution, then mix the binder solution with the sieved material, use a granulator to granulate, pass through a 24-mesh sieve and dry at 50°C.
[0052] The dried granules are passed through a 20-mesh sieve, the amount of lubricant is calculated and weighed, mixed with the dry granules, and compressed with a tablet machine to obtain tablets.
[0053] About tablet coating: 1) Prepare coating powder: hypromellose (65%, w / w, viscosity: 6cps), titanium dioxide (20%, w / w); talc ...
Embodiment 2
[0055] According to the method described in Example 1, benazepril hydrochloride and lercanidipine hydrochloride with a weight ratio of 10:3.3 were mixed with the following excipients to obtain tablets of the pharmaceutical composition of lercanidipine and benazepril.
[0056] Benazepril Hydrochloride
Embodiment 3
[0058] According to the method described in Example 1, benazepril hydrochloride and lercanidipine hydrochloride with a weight ratio of 10:3.3 were mixed with the following excipients to obtain tablets of the pharmaceutical composition of lercanidipine and benazepril.
[0059] Benazepril Hydrochloride
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com