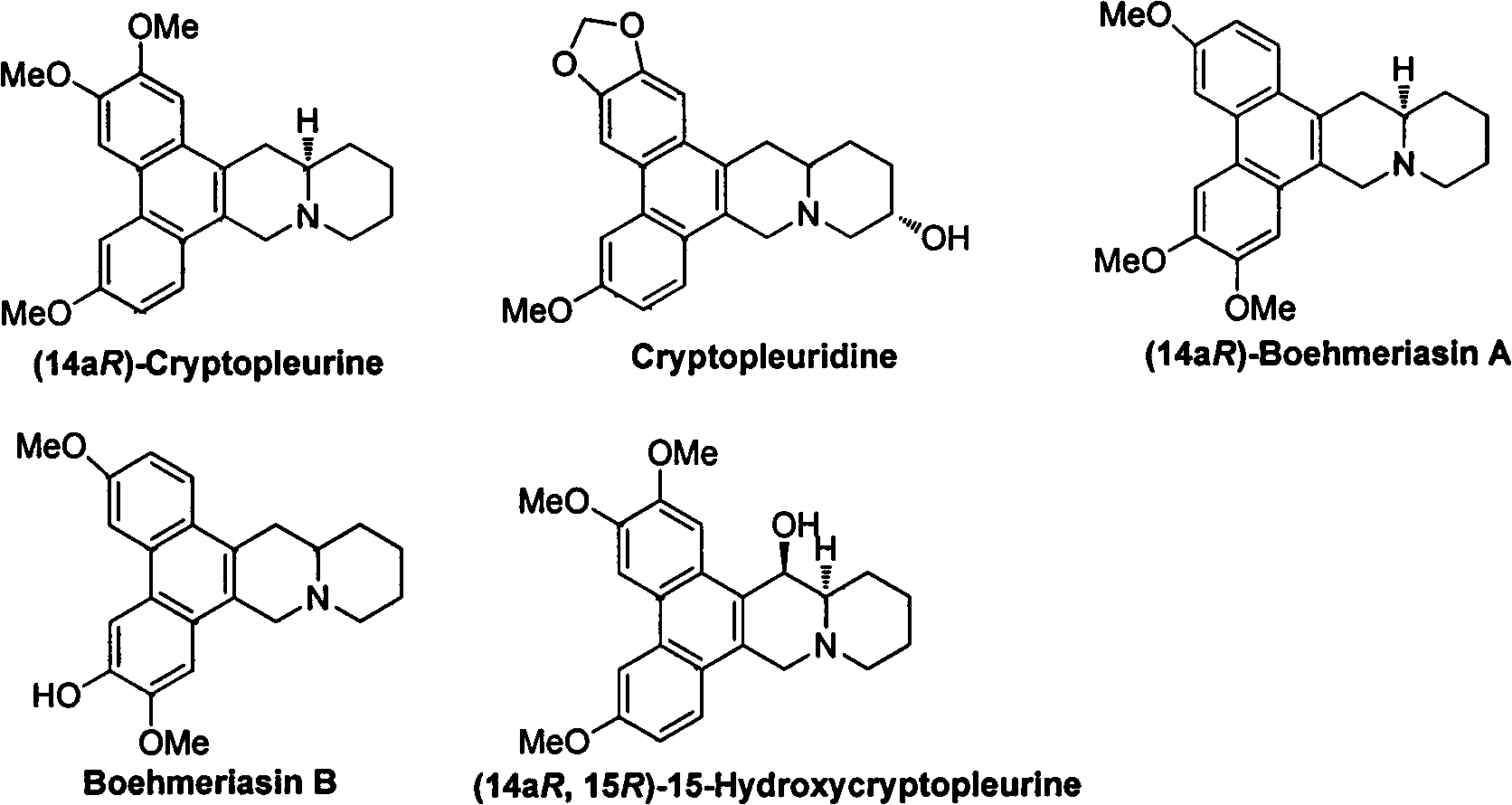
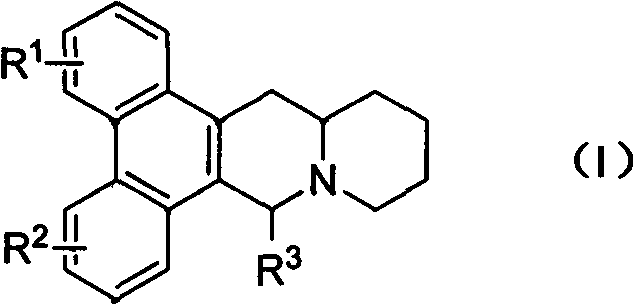
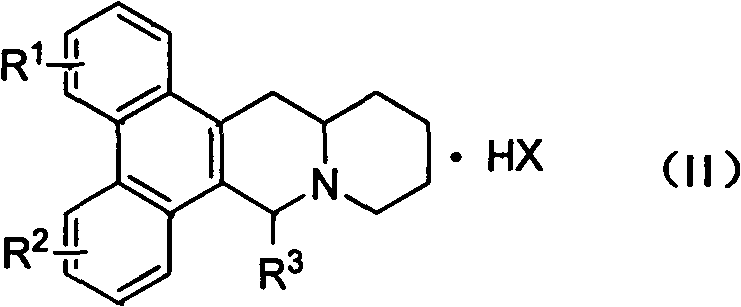
Phenanthroquinolizidine alkaloid derivatives and salts thereof, and preparation, anti-plant virus activity and anti-cancer activity of phenanthroquinolizidine alkaloid derivatives and salts thereof
A technology of alkaloid derivatives, phenanthroquinol, which is applied in the field of phenanthroquinolizidine alkaloid derivatives and their salts, and can solve the problem of unrepeatable, photothermal instability, and phenanthroquinolizidine derivatives. Single structure and other problems, to achieve good anti-cancer activity, excellent anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Synthesis of phenanthroquinolizidine alkaloid derivatives I-a-1~I-a-13
[0040] 2,3-Dimethoxy-6-benzyloxy-phenanthrene-[9,10-b]-quinolizidine (I-a-8) and 6-hydroxy-2,3-dimethoxy-phenanthrene -[9,10-b]-quinolizidine (I-a-9) synthesis:
[0041]
[0042]Synthesis of 2-(3,4-dimethoxyphenyl)-3-(4-hydroxyphenyl)methyl acrylate Add 19.6g (0.1mol) 3,4-dimethyl in sequence to a 250mL four-necked reaction flask Oxyphenylacetic acid, 12.2g (0.1mol) 4-methoxybenzaldehyde, 40mL acetic anhydride and 20mL triethylamine were stirred and heated for 20h. The mixture was cooled to room temperature, 60 mL of water was added thereto, reacted for 8 h, filtered, and washed with methanol to obtain a yellow solid, 22.1 g in total, and the above yellow solid, 600 mL of methanol, and 4 g of concentrated sulfuric acid were added to a 1 L four-neck flask, and heated Reaction 12h. The reaction solution was desolvated to obtain a brown solid, which was recrystallized with methanol...
Embodiment 2
[0082] Embodiment 2: Synthesis of phenanthroquinolizidine alkaloid derivatives I-b-1~I-b-7 and I-c-1
[0083] 2,3,6,7-Tetrabenzyloxycarbonyloxy-phenanthrene-[9,10-b]-quinolizidine (I-b-1) and 2,3,6,7-tetrahydroxy-phenanthrene- Preparation of [9,10-b]-quinolizidine (I-c-1)
[0084]
[0085] Synthesis of 2,3,6,7-tetrabenzyloxycarbonyloxy-phenanthrene-[9,10-b]-quinolizidine (I-b-1) Add 0.4g (1mmol) to a 250mL one-necked bottle 2,3,6,7-Tetramethoxy-phenanthrene-[9,10-b]-quinolizidine (I-a-7), 80mL dichloromethane, install constant pressure dropping funnel and drying tube, in Add 20mL of dichloromethane and 3mL of boron tribromide into the constant pressure dropping funnel, put the reaction bottle into a low-temperature bath, stir the reaction, and add the dichloromethane solution of boron tribromide dropwise. After the dropwise addition, react for 36h. Add methanol, spin out the reaction solution, add 60mL of water and 60mL of tetrahydrofuran to dissolve the residue, pour it...
Embodiment 3
[0101] Example 3: 2,3,6,7-tetramethoxy-phenanthrene-[9,10-b]-quinolizidine-9-formic acid ethyl ester (I-d-1) and 2,3,6, Preparation of 7-tetramethoxy-phenanthrene-[9,10-b]-quinolizidine-9-methanol (I-d-2)
[0102]
[0103] Synthesis of ethyl 2,3,6,7-tetramethoxy-phenanthrene-[9,10-b]-quinolizidine-9-carboxylate (I-d-1)
[0104] In a 100mL four-neck flask, add 0.8g (2mmol) 2-(2,3,6,7-tetramethoxy-9-phenanthrenylmethyl)-piperidine, 60mL acetonitrile, pass through argon protection, add 2.6 mL50% ethyl glyoxylate solution in toluene, add 0.1mL glacial acetic acid. The reaction was stirred at room temperature for 24h. Add saturated sodium bicarbonate solution for treatment, extract with dichloromethane, wash twice with saturated sodium bicarbonate, then wash once with saturated brine, add anhydrous sodium sulfate to dry, filter and precipitate to obtain a yellow solid 0.9g, yield 83.3%, melting point 167-169°C; 1 H NMR (400MHz, CDCl 3 )δ1.25(t, J=7.2Hz, 3H), 1.41-1.54(m, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com