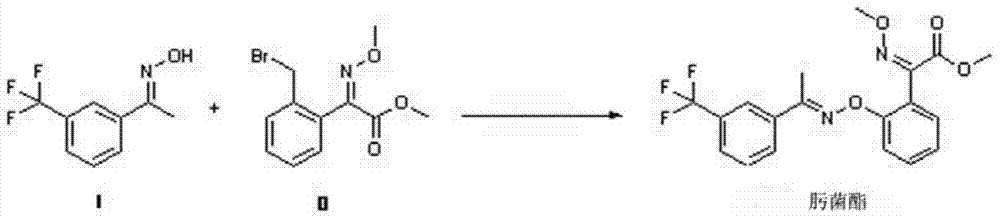
Synthesis method of trifloxystrobin
A synthetic method, the technology of trifloxystrobin, which is applied in the field of trifloxystrobin synthesis, can solve the problems of difficulty in industrial production, incomplete process, and difficulty in obtaining raw materials, and achieve the effects of improving product quality, avoiding cross-contamination, and reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add compound I (21.0 g, 0.100 mol, 97%) and sodium hydroxide (4.2 g, 0.100 mol, 95%) to the reaction flask equipped with a stirrer, water separator, condenser and thermometer after nitrogen replacement and toluene (45.0g), start stirring, heat to reflux to divide water, reflux for 4 hours, stop heating, cool down to room temperature, dissolve compound II (31.6g, 0.105mol, 95%) in toluene (20.0g ), the toluene solution of compound II was added dropwise to the above reaction solution, and added dropwise for 0.5 hours, kept at 30°C for 3 hours, then sampled and analyzed, and after the reaction was completed, the reaction was processed. Add water (50g) to the reaction solution, stir for 10 minutes, let stand, separate layers, wash the organic phase with water (wash twice, 50ml each time), the pH of the water after washing is 6, the organic phase cools down and crystallizes, and keep warm at 20°C for 1 Hours, part of the solid precipitated, kept at 10°C for 2 hours, kept at ...
Embodiment 2
[0022] Add compound I (21.0g, 0.100mol, 97%) and potassium hydroxide (6.2g, 0.100mol, 90%) to the reaction flask equipped with a stirrer, water separator, condenser and thermometer after nitrogen replacement and benzene (45.0g), start stirring, heat to reflux to divide water, reflux for 4 hours, stop heating, cool down to room temperature, dissolve compound II (31.6g, 0.105mol, 95%) in toluene (20.0g ), the toluene solution of compound II was added dropwise to the above reaction solution, and added dropwise for 0.5 hours, kept at 30°C for 3 hours, then sampled and analyzed, and after the reaction was completed, the reaction was processed. Add water (50g) to the reaction solution, stir for 10 minutes, let stand, separate layers, wash the organic phase with water (wash twice, 50ml each time), the pH of the water after washing is 6.5, the organic phase cools down and crystallizes, and keep warm at 20°C for 1 Hours, part of the solid precipitated, kept at 10°C for 2 hours, kept at...
Embodiment 3
[0024] Add compound I (21.0 g, 0.100 mol, 97%) and sodium hydroxide (4.2 g, 0.100 mol, 95%) to the reaction flask equipped with a stirrer, water separator, condenser and thermometer after nitrogen replacement and cyclohexane (45.0g), start stirring, heat to reflux to divide water, reflux for 4 hours, stop heating, cool down to room temperature, dissolve compound II (31.6g, 0.105mol, 95%) in cyclohexane Alkanes (20.0g), the cyclohexane solution of compound II was added dropwise to the above reaction solution, added dropwise for 0.5 hours, kept at 30°C for 3 hours, then sampled and analyzed, after the reaction was completed, the reaction was processed. Add water (50g) to the reaction solution, stir for 10 minutes, let stand, separate layers, wash the organic phase with water (wash twice, 50ml each time), the pH of the water after washing is 7.5, the organic phase cools down and crystallizes, and keep warm at 20°C for 1 Hours, part of the solid precipitated, kept at 10°C for 2 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com